With the promotion of functional plant oils, individuals are increasingly focusing on the health benefits and nutritional value of fatty acids [27,28]. This awareness could lead to more informed dietary choices that prioritize heart health and overall well-being [29,30,31,32,33]. The fatty acid composition of rapeseed has become a focal point for researchers in rapeseed breeding both domestically and internationally [34,35,36]. The fatty acid composition of seeds is regulated by a few key genes, but oil content involves multiple metabolic pathways [36]. This complexity highlights the challenges and opportunities in improving oilseed crops through genetic and metabolic engineering. Identifying the functions of genes involved in the fatty acid composition could provide an important theoretical basis for the breeding of functional seed oils. This knowledge could help to enhance the nutritional quality and health benefits of oilseeds, ultimately contributing to the development of improved varieties with desirable oil profiles.
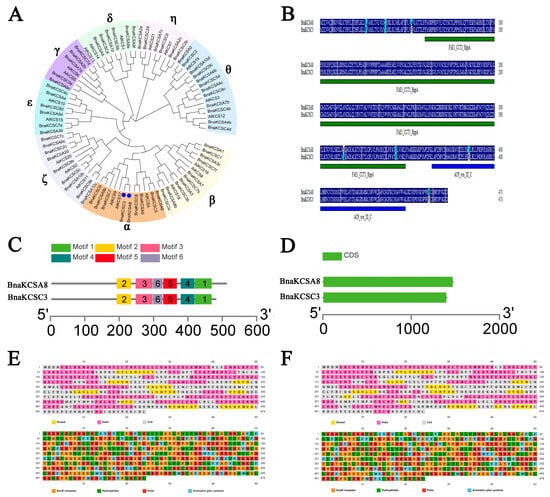
KCS is a key gene that has been proven to participate in fatty acid elongation, and its products can alter the composition of fatty acids. The fatty acid composition of rapeseed is closely related to the quality of the seed oil, so it is essential to isolate and identify the functions of BnaKCSes. Therefore, two BnaKCS family members, BnaKCSA8 and BnaKCSC3, were cloned and identified in Brassica napus. Through genetic evolution and bioinformatics analyses, it has been found that these two members have strong similarity and belong to the α-group of the KCS family, indicating a close phylogenetic relationship. These two members encode 474 aa. An analysis of the protein domains of the BnaKCSes through the SMART database revealed that they both contained similarly located ACP_syn_III_C and FAE1_CUT1_RppA domains (Figure 1B). This is consistent with the findings of Sagar regarding the conserved domains of KCS family proteins, indicating that the two genes identified in this study belong to the KCS gene family [26]. The gene structure and motif analysis results show that both genes contain the same motifs, and the number of introns and exons is consistent (Figure 1C–D). Given the striking similarity in the structures of these two genes, it is inferred that they may play similar roles in fatty acid biosynthesis. We also conducted subcellular localization studies on BnaKCSA8 and BnaKCSC3 using Arabidopsis protoplasts. The results show that both genes are localized in the endoplasmic reticulum (Figure 2), which is consistent with the subcellular localization results for KCS [21].
To investigate the roles of these two BnaKCSes in the fatty acid composition, we first conducted preliminary validation in yeast cells to determine whether the BnaKCSes could alter the composition of fatty acids. Compared to the control, the content of OA (C18:1) and PA (C16:0) were significantly increased in the pYES2-A8 transgenic yeast cells. In the pYES2-C3 transgenic yeast cells, the content of OA (C18:1) decreased (Figure 4), indicating that both genes are capable of altering the fatty acid composition. This is consistent with the results observed for peanut AhKCS, which also changed the composition of fatty acids when expressed in yeast [11]. In Camelina sativa, the heterologous expression of the Lunaria annua KCS also altered the fatty acid composition [37]. Additionally, we overexpressed the BnaKCSes in Arabidopsis to further validate their function. In OEA8, the levels of PA (C16:0) increased by 16.3% and linolenic acid (C18:3) by 11.6%, EA (C22:1) decreased by 5.5% (Figure 5C). This indicates that the introduction of BnaKCSA8 altered the composition of fatty acids. This is in line with results showing that KCS can alter the fatty acid composition in Arabidopsis seeds [17,38]. In OEC3, the levels of linolenic acid (C18:3) increased by 18.5%, in contrast, the levels of PA (C16:0) and EA (C22:1) decreased by 9.6% and 5.9% (Figure 5C). This is consistent with results regarding the overexpression of CsKCS6, which led to changes in the fatty acid composition [39]. The fatty acid components altered by BnaKCSes in yeast and Arabidopsis were different, which may be due to the difference in the substrate required by these two genes, or some other reasons, which need further study. In this study, these two genes can change the fatty acid composition. Oil content analysis showed that the oil content of transgenic plants was lower than that of wild type (Figure 5D), which was similar to the results of BnFAE1 [23]. Although overexpression of a gene involved in fatty acid biosynthesis is expected to increase oil content. However, because the oil content is affected by many factors, the changes of fatty acid components are not necessarily positively correlated with the oil content.
Research by Kim et al. has shown that KCS interacts with KCR1, PAS2, and ECR to regulate fatty acids [21]. To determine whether other genes respond to fatty acids, we also examined the expression levels of additional genes associated with fatty acids in transgenic plants. Compared to the WT, AtKCR1, AtCER10, and AtWRI4 were upregulated in the OEA8 line (Figure 5E). This result is consistent with the findings that the overexpression of BnWRI1 increases the fatty acid content and that the upregulation of AtWRI transcription factors promotes oil accumulation [40,41].
Source link
Dongfang Zhao www.mdpi.com