1. Introduction
Plants, as sessile organisms, must respond effectively to environmental stimuli to achieve optimal development. Different environmental factors have the potential to inflict stress on plants, limiting their growth. Abiotic stresses, manifested in different ways, are frequently associated with changes in climatic conditions, such as rain, temperature, solar irradiation, and soil quality. Changes in water availability and temperature, leading to hydric and heat stresses, have been related to climate change’s impact [
1]. The integration of models on climate change and agricultural production considers that, soon, the abrupt impact of changes in environmental conditions will intensify, representing a greater risk to the productivity of crops. Given plants’ crucial role as a primary food source, this situation could have serious implications for food security in a rapidly growing global population. Moreover, plants are important fuel and fiber sources, contributing significantly to production diversity and ecological sustainability [
2,
3].
Through evolution, plants have developed adaptive strategies to respond to environmental challenges to ensure their reproductive success. These strategies include a series of morphological, anatomical, and metabolic adjustments to better cope with the environmental conditions. Among these strategies is the crucial importance of reactive oxygen species (ROS) as secondary messengers as well as the signaling mediated by phytohormones, which perform an essential function in plant development and reproduction [
4,
5]. The integration between hormonal signaling and the management of ROS homeostasis controls diverse types of signaling, especially those involved with stress responses [
6].
Many of the signaling pathways in which ROS act as signaling molecules are orchestrated by phytohormones [
7], which play an indispensable role in metabolism, development, coordination, stress response, and even in death [
8]. Plants synthesize various phytohormones, such as abscisic acid (ABA), ethylene (ET), salicylic acid (SA), jasmonic acid (JA), gibberellins (GA), auxin (AUX), cytokinin (CK), brassinosteroids (BRs), and strigolactones (SL), to orchestrate and regulate various aspects of growth and development. These phytohormones play a fundamental role in a myriad of dynamic yet rigorously regulated molecular responses throughout the plant life cycle. Responding to environmental adversities, these phytohormones play a crucial regulatory role in gene expression machinery, preparing the plant to withstand unforeseen circumstances [
9,
10,
11].
Among the different types of abiotic stress, drought emerges as the main factor that harms crops globally and directly impacts harvests, ultimately restricting total yield potential [
12]. The primary mechanisms controlling drought response involve stomatal closure, modulation of root growth and architecture, and the upregulation of anti-stress proteins [
13]. As additional strategies that contribute to mitigating the adverse effects of water stress, cells reduce their hydric potential (ψ), leading to the accumulation of specialized solutes. All these multivariate responses are mainly orchestrated by various hormonal regulations, which support cellular plasticity and help plants develop an effective coping strategy against drought [
14]. During water shortage, phytohormones regulate plant growth and development, resulting in increased antioxidant enzyme production, secondary metabolites, and heat-shock proteins [
15]. Several studies have indicated that communication between ROS and phytohormones signaling is essential to initiate and modulates the response of tolerance of different stresses, including drought. Therefore, precise coordination in the interaction with phytohormones is essential for the appropriate response in plants [
7].
To develop comprehensive concepts and strategies for safeguarding plants against the deleterious effects of abiotic stress and meeting the future demands for plant products, an in-depth exploration of molecular-level mechanisms governing plant stress responses is imperative. This review focuses on elucidating the intricate roles played by ROS, redox metabolism, and signaling mediated by the different phytohormones classes—abscisic acid, ethylene, salicylic acid, jasmonic acid, gibberellins, auxin, cytokinin, brassinosteroids, and strigolactones—in the context of drought stress. The objective is to provide a comprehensive overview of the existing knowledge about how these signaling cascades and redox metabolic processes synergize, leading to robust responses to water stress in plants.
11. Phytohormones Crosstalk During Drought Response
Although ABA is recognized as the main phytohormone related to drought, the outcomes of response and tolerance to adverse conditions result from a complex interaction of multiple hormonal actions [
533]. The stress response pathways exhibit intricate connections, often converging on common elements, which is referred to as “crosstalk”. The term crosstalk denotes situations where different signaling share one or more elements, resulting in shared outputs [
534] (
Figure 10). The plants’ physiological responses to stressful environments, involving changes in biological processes, arise from antagonistic or synergistic interactions between various phytohormones [
535]. Consequently, the development of tolerance in plants against drought emerges as a complex phenomenon involving intricate interactions across various cellular, molecular, and metabolic dimensions [
8].
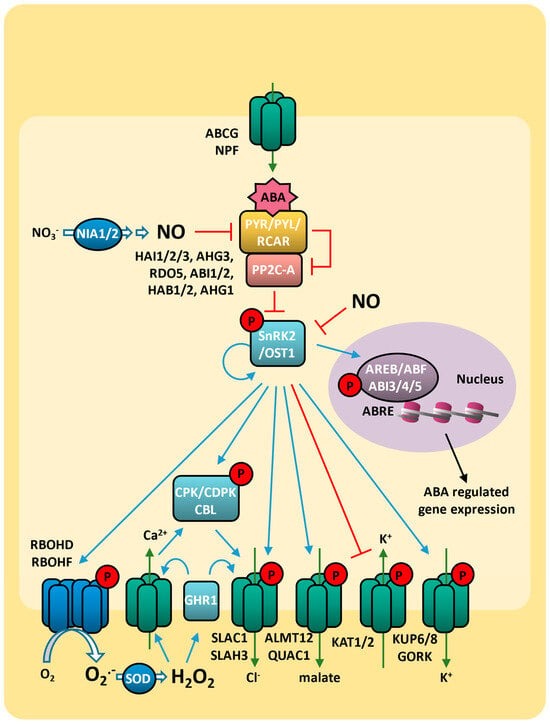
The ABA regulation of stomatal closure under abiotic stress is directly related to ethylene signaling, which can act as either a positive or negative regulator [
101]. This mechanism is dependent on ABA concentration. When ABA is not sufficient, drought stress leads to ethylene synthesis, which then induces the accumulation of ROS synergistically with ABA, inducing stomatal closure through the synthesis of NO and the activation of the SLACs channel [
98,
536]. In this condition, ethylene is also able to induce ABA biosynthesis through ERF transcription factors. In different species, the overexpression of
ERFs results in increased ABA content, rapid stomal closure, and enhanced abiotic stress tolerance [
101]. On the other hand, ABA inhibits
ERF1 gene expression through a negative-feedback mechanism [
537]. When ABA levels are high, ethylene starts to have an oppositive effect, inhibiting ABA-induced stomatal closure [
98]: both exogenous ethylene and the ethylene-overproducing mutant
eto1-1 show a reduced ABA-induced stomatal closure in drought-stressed arabidopsis [
538]. It has been suggested that, under these conditions, ethylene might trigger the production of flavonoids, which could decrease NADPH oxidase activity. This would subsequently inhibit the synthesis of ROS induced by ABA, ultimately preventing ABA-induced stomatal closure [
539,
540]. Ethylene also regulates ROS metabolism by modulating antioxidant enzymes such as SOD, CAT, APX, and GR [
541,
542]. Similarly, the ethylene precursor ACC induces APX, CAT, SOD, and POX activities and reduces lipid peroxidation [
543].
Despite the initial observation of water stress-induced ethylene synthesis [
544], some studies have yielded varied and sometimes contradictory results, suggesting that drought stress either fails to increase or reduces ethylene production [
545]. The ABA-induced transcription factor ABI4 mediates the transcriptional repression of
ACS4 and
ACS8, resulting in inhibition of ethylene biosynthesis during elevated ABA levels [
98]. This is supported by the increased ethylene production in maize and tomato mutants deficient in ABA synthesis [
546,
547]. The combined ethylene–ABA stimulus results in half-open stomata, with diminished closure compared to the effect of the individual ABA or ethylene stimulus [
548]. In this context, it was demonstrated that the inhibition of ethylene biosynthesis and perception can increase stomata closure, enhancing drought tolerance. In maize, the silencing of ACS decreases ethylene biosynthesis and improves drought tolerance [
549]. Similarly, plants with reduced ethylene sensitivity, such as arabidopsis and maize overexpressing
ARGOS genes [
74] and arabidopsis mutants
etr1-1 and
ein2-1 [
550], showed reduced water loss through transpiration and increased drought tolerance.
Despite this, reduced stomatal conductance and transpiration are not directly associated with drought tolerance, and the limitation of stomatal conductance is recognized as the primary factor contributing to the reduction in photosynthesis and oxidative stress during drought response [
551,
552,
553]. Although reduced stomatal conductance leads to lower transpiration and water loss, it also restricts the supply of CO
2 for RubisCO (EC 4.1.1.39) carboxylase activity, thereby limiting photosynthesis and diverting ribulose bisphosphate (RuBP) to photorespiration, which is the primary source of ROS in photosynthetic tissues [
551,
552,
553]. Therefore, the regulation of ABA-induced stomatal closure, along with the activation of the antioxidant system through the ethylene signaling, plays a central role in ensuring an appropriate response to drought (
Figure 10).
Under moderate drought conditions, ABA can positively regulate root system architecture, increasing primary root length and root hair density through its induction of auxin biosynthesis and transport in the primary roots [
554,
555,
556,
557]. Indeed, the knockout of the ABA catabolic gene
ABA8ox in rice [
556] or the exogenous ABA application in arabidopsis [
555] increases the auxin flux towards the root, resulting in increased root length and drought tolerance. Low concentrations of ABA also facilitate primary root growth by mitigating PP2C-D-mediated inhibition of the apoplastic efflux of H
+ through the AHA2 channel [
558]. For effective hydrotropism, the ABA-induced kinase SnRK2.2 is required at the cortical cells in the root elongation zone, where it promotes cellular elongation, allowing differential growth [
559]. On the other hand, at elevated concentrations, ABA inhibits root growth by disrupting auxin signaling in roots, repressing genes encoding transporters responsible for auxin translocation [
560]. Taken together, these observations show that both auxin and ABA play crucial roles in shaping the root system architecture in response to water availability. Mild soil drying and low ABA concentrations stimulate root growth by inducing auxin signaling. However, as drought conditions intensify, and ABA and ROS levels increase, auxin signaling and root growth are suppressed. While this inhibition does not enhance water absorption, it serves as an adaptive strategy during severe drought, allowing plants to allocate resources toward preserving photosynthetic tissues, thereby maximizing their chances of survival [
561].
ABA and brassinosteroids are known to act antagonistically, balancing growth and stress response. BIN2 kinase seems to be the central hub of this interaction since its activity is repressed by dephosphorylation mediated by PP2Cs, a part of the central core of ABA signaling [
476,
562]. Under drought conditions, the increase in ABA leads to the repression of the PP2Cs ABI1 and ABI2. This allows BIN2 to be an active kinase, attenuating growth responses due to the phosphorylation of BES1/BZR1 transcriptional factors, which reduces the transcription of the brassinosteroid-induced genes related to growth [
476]. On the other hand, BIN2 also phosphorylates SnRK2s, intensifying ABA signaling [
475,
563]. ABA also negatively regulates the expression of the transcriptional factors BEE1, BEE2, and BEE3 [
464].
Pioneer analyses of auxin and brassinosteroid co-regulation have demonstrated that the signaling of both hormones converges at the promoters of shared target genes [
462,
564]. BES1 and BZR1 also integrate brassinosteroid signaling with other phytohormone signaling, co-regulating plant growth and stress tolerance [
565,
566,
567,
568]. Promoter regions from several transcriptional factor genes involved in light, auxin, and gibberellin signaling are targets of BZR1 [
460]. BES1 and BZR1 also interact with PIF and DELLA transcriptional factors, coregulating several genes and modulating cell elongation and photomorphogenesis [
456,
569,
570,
571].
The presence of jasmonic acid in the roots has been suggested as essential during water stress to increase ABA levels [
27,
572]. Despite this, jasmonic acid inhibits root growth and reduces meristem activity [
573]. This inhibition appears to be mediated by the interaction between jasmonic acid and auxin signaling [
574]. Upon jasmonic acid induction, MYC2 binds to the promoters of the auxin-responsive gene
PLT (PLETHORA), responsible for stem cell niche maintenance and cell division, leading to suppression of its expression and thereby inhibiting root meristem activity [
574]. On the other hand, jasmonic acid inhibition of root growth is negatively regulated by brassinosteroids [
575], which act antagonistically to jasmonic acid during plant defense [
576]. The antagonistic regulation of metabolic genes is the main feature of gibberellin and ABA interactions [
574]. ABA-deficient mutants exhibit elevated gibberellin levels, highlighting ABA’s role in suppressing gibberellin metabolic genes (
GA3ox1/2 and
GA3ox1/2/3) during seed germination. Conversely, gibberellin-deficient mutants show increased ABA levels by upregulating ABA biosynthetic genes (
ABA1,
NCED6, and
NCED9) while downregulating the ABA catabolic gene (
CYP707A2) [
577,
578]. Under water-limited conditions, gibberellin can inhibit ABA biosynthesis and stomatal closure [
579]. Gibberellin is also recognized to antagonize ABA regulation of different developmental stages, including seed dormancy, seed germination, root growth, leaf development, flowering time, and responses to environmental cues such as light, temperature, and other abiotic stresses [
580].
The gibberellin signaling appears to impair stress responses. The DELLA protein family, which inhibits gibberellin signaling in its absence, also promotes an ABA response by promoting the expression of the ABA transporter gene
ABA-IMPORTING TRANSPORTER 1.1 (
AIT1.1) in guard cells [
540] and activating ABI transcription factors [
581]. Transgenic tomato plants overexpressing the constitutively active DELLA protein proceraΔ17 (proΔ17) showed decreased stomatal aperture and plant transpiration; however, these effects are suppressed in the ABA-deficient
sitiens (
sit) mutant, suggesting DELLA’s role is ABA-dependent [
241]. Similarly, DELLA can bind and inhibit JAZ, the central negative regulator of jasmonic acid signaling [
582]. Gibberellin-mediated degradation of DELLA contributes to improve JAZ inhibition of jasmonate-responsive gene expression [
583,
584] and jasmonate-mediated plant immune responses [
585].
Despite antagonizing the stress-induced ABA and jasmonate responses, gibberellin acts synergically to auxin, brassinosteroids, and ethylene, exerting a pivotal role in the modulation of intricated molecular and cellular processes that allow plants to respond to environmental conditions [
239]. Gibberellin can regulate the auxin transport by inducing the expression of the PIN-FORMED auxin transporters
PIN1,
PIN2, and
PIN3. This mechanism has an important role in gravitropism and xylogenesis [
294,
586]. Indeed, arabidopsis gibberellin biosynthesis- and signaling-deficient mutants show reduced activity of PIN, and the wild-type phenotype is restored upon exogenous gibberellin application [
587]. Additionally, DELLA protein RGA interacts with and inhibits the auxin-responsible factors ARF6, ARF7, and ARF8. Gibberellin enhances the transcription of auxin response by promoting DELLA degradation [
568]. Similar mechanisms appear to allow gibberellin to positively regulate the transcription of brassinosteroids and ethylene-responsive genes. DELLA also regulates BES1 and BZR1 1transcription factors, which control the responses and are activated by gibberellin via DELLA degradation [
588], indicating an important intersection between gibberellin and brassinosteroids signaling. In ethylene signaling, the degradation of DELLA mediated by gibberellin is accompanied by the inhibition of the stress-responsive genes
AP2/ERF [
239]. Additionally, gibberellin induces ethylene biosynthesis by inducing
ACS5 and
ACS8 [
589].
Stress-induced ABA accumulation downregulates cytokinin biosynthesis through the MYB2 transcription factor, which relieves the repression of cytokinin signaling and activates ABA- and stress-inducible genes [
590]. Indeed, ABA and cytokinins regulate bud outgrowth antagonistically [
591]. Under drought conditions, tZ transport is impaired [
592]. Key kinases in the ABA signaling, such as SnRK2.2, SnRK2.3, and SnRK2.6, directly phosphorylate several Ser residues of ARR5, a type-A ARR and inhibitor of cytokinin signaling. This phosphorylation stabilizes the ARR5 protein, enhancing drought tolerance by suppressing cytokinin signaling and by positively regulating ABA signaling in an SnRK2-dependent manner [
593]. Despite acting antagonistically, during biotic stress response, cytokinins induce ROS production and stomata closure. This mechanism is not affected in the ost1-3 mutant, indicating that it occurs in an ABA-independent manner [
160]. In arabidopsis, both ABA and drought downregulate the expression of
ARR1,
ARR10, and
ARR12 [
397]. Additionally, ABA-activated ABI4 binds the promoters of
ARR6,
ARR7, and
ARR15, further impairing their expression [
594,
595,
596]. Thus, the ABA-mediated inhibition of cytokinin signaling can reshape the plant body by downregulating shoot growth while accelerating root growth. This allows the plant to reduce water loss and increase water uptake from deeper soil layers [
590].
Conversely, elevated endogenous cytokinins suppress ABA signaling, thereby influencing a trade-off between growth and defense mechanisms. This reduced sensitivity to ABA under high cytokinins levels is thought to be mediated through type-B ARRs such as ARR1, ARR11, and ARR12, which interact directly with SnRK2s, inhibiting the kinase activity of SnRK2.6 [
593]. Indeed,
AHK2,
AHK3, and
AHK4 defective mutants as well as type-B ARRs
ARR1,
ARR10, and
ARR16 exhibit higher sensitivity to ABA and display increased resistance to drought [
397,
597,
598]. On the other hand, the type-A ARRs ARR4, ARR5, and ARR6 downregulate ABI5 expression, contributing to ABA response [
595].
Strigolactones act as positive regulators of stress signaling networks and various ABA signaling by regulating the expression of many stress- and ABA-responsive genes involved in plant development and abiotic stress responses. Impaired strigolactone signal transduction also leads to the downregulation of CKX genes, which are necessary for cytokinin degradation [
527]. These findings suggest that coordinated crosstalk between strigolactones, ABA, and cytokinins signaling networks regulates plant adaptation to adverse environmental conditions. Consistent with the drought-sensitive phenotype, max mutants, which show disrupted strigolactone biosynthesis, displayed increased leaf stomatal density compared to wild-type plants and exhibited slower ABA-induced stomatal closure. Relative to the wild type, max mutants showed a higher rate of leaf water loss during dehydration and reduced ABA responsiveness during germination and post germination, underscoring the role of strigolactones as positive regulators of ABA signaling [
527].