1. Introduction
The steam reforming process is that which leads to the production of gray hydrogen, and it is, therefore, preferred over other processes that could lead to pollution and environmental deterioration, hence the interest of researchers in this area [
1]. Hydrogen being a promising energy solution for the future, coupled with its renewability and eco-friendliness, has been predicted to replace fossil fuels, which are known for their role in the deterioration of the ecosystem [
2]. Some conventional ways of producing hydrogen include from fossil fuel, which is achieved by the steam reforming of natural gas or from heavy fuel oil via partial oxidation [
3]. Since the utilization of fossil fuels is not suitable for the environment [
4], researchers have embarked on the search for alternatives to produce alternative fuels by steam reformation using a couple of chemicals, namely; ethanol [
5,
6,
7], glycerol [
8,
9,
10], methanol [
11,
12,
13], acetic acid [
14,
15,
16], and acetone [
14,
15,
17]. Different routes exist for the production of hydrogen gas from alcohols and organic acids.
Acetone steam reforming is one alcohol reforming process that is very promising and not much work has been done on it [
18]. Another strong justification for the production of hydrogen from acetone is that acetone is not a valuable product itself, as it is not in high demand, so it can be upgraded to a much more valuable product: hydrogen [
2]. Whilst phenol, the primary product in cumene peroxidation, is in high demand globally—to the tune of 12.5 million tons per annum—demand for its by-product, acetone, is only 6.8 million, whereas the production capacity is 8.5 million tons [
1]. This obvious gap needs to be filled by the conversion of the excess acetone to hydrogen via steam reforming reactions. The likely reactions occurring during the reforming process are shown Equations (1)–(8).
Hydrogen is an environmentally friendly fuel that can be applied in highly effective systems, such as fuel cells for the generation of renewable energy [
4]. This is because the chemical reaction between hydrogen and oxygen in a fuel cell will give only water as its product, thus keeping the environment clean and free from pollution of any kind. Basu and Pradhan studied the selective synthesis of hydrogen using acetone steam reforming over a Ni-Co/olivine catalyst that exhibited a significant steam reforming performance of up to 99% conversion of acetone and around 80% catalyst selectivity [
2]. The studies by Braga et al. showed that the acetone conversion process on a Co, Co-Ni, or Ni catalyst depended on the nature of metals, the temperature of the reaction, the metal oxidation state on the nanoparticles’ surface atoms [
4], and that acetone decomposition via reduced metals at a temperature >350 °C took place via the breaking of H-C and C-CO bonds and the formation of C, CO, and H
2 on the metal surface. Esteban-Diez et al. produced a blend of acetone and acetic acid which was achieved in a fluidized bed reactor and gave a yield of 83.3–88.6% which is comparable to the individual yield for hydrogen gas [
3]. Paschenko studied the application of Aspen HYSYS in achieving a thermodynamic comparison of low-grade heat utilization via steam generation and methanol decomposition in which it was observed that the latter was 5% more efficient than the former [
19]. Paschenko and Mustafin also performed thermodynamic studies on thermochemical recuperation (TCR) systems, where it was confirmed that, within a range of temperatures from 150 to 500 °C, there were heat deficits that were addressed by condensing about 15% of fuel gas through a condenser after the preheater arrangement was aided by Aspen HYSYS software [
20]. The thermodynamics of a combined cycle power plant (CCPP) were compared with that of a chemically recuperated gas turbine (CRGT) by Paschenko with the aid of Aspen HYSYS, and it was discovered that the latter performed better, with an efficiency value 4–7% higher [
21]. Also, Ighalo and Adeniyi modeled the pyrolysis, in-line stream reforming, and air gasification of switchgrass using ASPEN Plus v8.8 and found that, at an optimum steam reforming temperature of 700 °C, 1 atm of pressure, and a 10 kg/kg steam-to-feed ratio, 70.23 mol% of H
2 was produced [
22].
Despite much work on traditional catalysis investigations in acetone steam reforming, a pure component thermodynamics analysis using the Soave–Redlich–Kwong (SRK) equation of state (EOS) has not been reported, according to the author’s exhaustive search. Several studies have used other EOS approaches [
23,
24] to adjust singlet or mixture reforming processes [
14,
25,
26]. There is a need to develop pure component data for factor effects and interactions, as this provides researchers with contributions of these components to observe process responses in real systems. Thermodynamics analysis of pure components is important because it allows for the fundamental understanding of factor effects based on an equation of state during the reforming process. This study aimed to utilize the Gibbs free energy minimization method to conduct a thermodynamic analysis of the steam reforming process for acetone on Aspen Plus v8.8. This study unveiled the need to balance hydrogen production with the reverse gas shift reaction by operating at the threshold of these optimum parameters: 600 °C, STOR of 12, and atmospheric pressure for an optimum hydrogen yield of 70 mol%. From a practical standpoint, the study was limited only to steam reforming of pure component acetone without reference to catalysis, as thermodynamic calculations do not generally capture that.
2. Results and Discussion
The idealistic effect of key factors on the performance of the acetone steam reforming system can be determined by carrying out the thermodynamic analysis of the process. This can be achieved by evaluating the effect of process variables (temperature, pressure, and STOR) on the selectivity of the product species H
2, CO, CO
2, and CH
4. Most workers are interested in the heterogeneous alternative of the process, whose results, as expected, showed higher conversion and selectivity for H
2 production from acetone [
1,
2,
4,
18].
2.1. Effect of Process Temperature
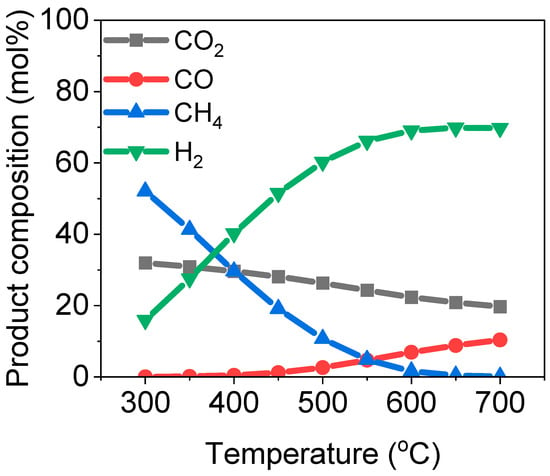
The steam reforming process is a highly endothermic reaction that progresses with an increase in temperature. It can be observed from
Figure 1 that there was a continuous rise in the amount of hydrogen with an increase in temperature. Similar observations have been made, albeit for glycerin steam reforming, by Wang et al. [
27]. Also, Basu and Pradhan made a similar observation with an Ni-Co/olivine catalyst for an acetone steam reforming process [
2]. Again, the gradual decrement in the amount of hydrogen produced at 650 °C could be due to other competing reactions taking place alongside the steam reforming of acetone, which includes the exothermic reverse water–gas shift reaction (2) that uses up part of the produced hydrogen to yield more water [
22]. This is a serious DRM challenge, as the amount of oxygen species present in the reaction environment can be very high. A similar observation was made for the in-line steam reforming of biomass pyrolysis vapors from switchgrass [
22]. Pyrolysis vapors from biomass tend to have a high O/C ratio due to the nature of biomass. The sharp drop in the amount of methane produced is largely due to the exothermic nature of the methane production reaction, which will be enhanced at lower temperatures [
28]. Generally, CO
2 is only by-product of a water–gas shift and methane reformation reaction, and hence, will need to be removed from the product stream via an adequate separation mechanism. At the optimum temperature condition of 600 °C, a STOR of 12, and an atmospheric pressure of 1 atm, the optimum amount of hydrogen produced was around 70 mol%. A temperature of 600 °C is considered optimum because it is a sweet spot that is high enough to favor high hydrogen production and low enough to limit the effect of the reverse water–gas shift reaction. This result is comparable to that of the heterogeneous alternative (Ni-Co/olivine), where, at a STOR as low as 6, under atmospheric pressure, and at a temperature of 500 °C, the selectivity of hydrogen plateaued at 80% [
2]. This finding offers insight that the catalyst was provided the required surface area for the reaction to proceed efficiently against the homogenous approach. Also, the space–time concept associated with the heterogeneous process suggests that the reactants should be allowed sufficient contact with the catalyst surface to enable an optimum result. This explains why the yield obtained in the present study was lower, even around comparable optimum conditions.
2.2. Effect of Steam-to-Oil Ratio
The effect of the steam-to-oil ratio (STOR) on the production of hydrogen and other by-products of the steam reforming reaction of acetone performed at 600 °C and at atmospheric pressure (1 atm) is shown in
Figure 2. The STOR is the measure of the amount of steam fed into the system for the reaction, and it determines the efficiency of reactant utilization [
28]. From the figure, it is evident that the optimum amount of hydrogen (70 mol%) was obtained at a STOR of 12, and the minimum (25 mol%) at a STOR of 1. This is the case because a high STOR will shift the equilibrium of the water–gas shift reaction forward, leading to a large amount of hydrogen gas, which is the desired product [
29,
30]. This is because steam is one of the reactants in the primary reactions (1); hence, there is a greater equilibrium shift towards the RHS, and all acetone is essentially consumed. Also, the equilibrium of the water–gas shift reaction is favored in the direction of hydrogen production. More steam favors the production of more CO
2 and reduces the production of CO around a STOR value of 3. The optimum value of the STOR was 12 at a temperature of 600 °C, and pressure of 1 atm. Though it is plausible to use a higher STOR, it does not affect the equilibrium much, as the maximum of the hydrogen curve in
Figure 2 is approached at a high STOR. This result compares well with the heterogeneous (NiCoMgAl) approach adopted in the work of Basu and Pradhan, who found that, at a STOR of 8 and optimum temperature of 500 °C, the selectivity for hydrogen production was 80% [
1]. This slight difference may be explained from the standpoint of an insufficient contact time for the reaction to proceed efficiently.
2.3. Effect of Process Pressure
The effect of pressure on the steam reforming process of acetone at 600 °C and STOR of 12 is shown in
Figure 3. It can be seen from the figure that the optimum hydrogen production (70 mol%) of the steam reforming process was at atmospheric pressure, and a decrement was recorded as the pressure increased. This is because the equilibrium of gas phase reactions shifts in favor of lighter components at lower pressures [
27,
28]. An increase in the pressure will lead to an increase in the production of heavier components in the reaction system and, consequentially, a decrease in the production of hydrogen, which is the lightest component. We noticed an increase in the methane fraction in the product at higher pressures. There were some slight changes in CO and CO
2 by-product concentrations in the product with a pressure increase. This is due to the complex effect of pressure on the equilibrium of the multiple side reactions. Most thermo-catalytic hydrogen production processes are favored at low pressures [
31,
32,
33,
34]. Here, there seems to be good agreement with both the pure component approach and the heterogeneous alternative in that both proceed and are favorable at low pressures. This is expected, as the concentration of the gas mixture will increase at high pressures, leading to unwanted products and a high energy penalty.
2.4. Interplay of Thermodynamic Understanding and Catalysis
The observations/findings in this study are primarily controlled by the thermodynamics of the acetone steam reforming reaction. In this section, we discuss how this understanding relates to actual catalytic acetone reforming. In catalysis, one of the key elements is the reaction rate, and this controls the kinetics. Among the multiple reactions occurring simultaneously, reactions with higher rates will shift the selectivity in favor of their products, as opposed to other competing reactions. In a reaction system with irreversible reactions, selectivity can be well described using kinetics. For systems with competing reversible reactions, the selectivity becomes complex, as the equilibrium of each reaction is dynamic and the product of one reaction can be a reactant in another side reaction (Equations (1)–(8)). Furthermore, some of these reactions are endothermic in the forward direction and others are exothermic in the forward direction; hence, the overall temperature effect is not explicit. Thermodynamic models are thus used to elucidate selectivity based on the Gibbs free energy of each reaction. The more negative the change in the Gibbs free energy, the more favorable the reaction is. Using this understanding, we can predict the product selectivity of these multiple reversible reaction systems with a high degree of accuracy and show how temperature, pressure, and feed ratios affect it.
4. Conclusions
This study investigated the thermodynamic analysis of steam reforming of acetone to produce hydrogen gas and some by-products. The steam reforming process was temperature dependent, with increasing temperatures leading to higher hydrogen production. Competing reactions, particularly the exothermic reverse water–gas shift, impacted hydrogen yields beyond 650 °C, emphasizing challenges in this highly oxidative reaction environment.
The study identifies 600 °C as the optimum temperature, striking a balance between maximizing hydrogen production and minimizing the reverse water–gas shift’s impact.
The STOR significantly influenced hydrogen and by-product production in the steam reforming of acetone. The optimal hydrogen yield (70 mol%) was achieved at a STOR of 12. High STOR values shifted the equilibrium of the water–gas shift reaction towards hydrogen production due to increased steam, effectively consuming acetone and favoring the desired product.
Optimal hydrogen production occurred at atmospheric pressure, with a decline observed as pressure increased. Higher pressures favored the heavier components in gas phase reactions, leading to an increase in heavier components and a consequent decrease in hydrogen, the lightest component.
The main limitation of this study was the idealistic understanding of the factor effects and interactions without recourse to catalysis. Typically, the concept of heterogenous catalysis is not captured in Gibbs free energy minimization calculations. This means the effects of various catalyst types are usually not studied. However, this information is useful in understanding how the pure components will affect equilibrium hydrogen yields in mixtures such as biomass pyrolysate and biomass bio-oil. Pure component acetone is also shown to be usable for hydrogen production through the steam reforming process; hence, this study lays an important foundation in that area.