1. Introduction
Maternal stress can expose the developing fetus to high glucocorticoid levels by crossing the placental barrier, resulting in lifelong effects on physiology, immune function, and behavior in the progeny [
1,
2]. More specifically, exposure during fetal development can affect the hypothalamic–pituitary–adrenal (HPA) axis, resulting in postnatal changes in behavior and stress responsiveness of the progeny [
3,
4]. In pigs, high maternal cortisol exposure can induce postnatal changes in the corticotropic axis, indicating that prenatal stress effects may be partly due to changes in this axis [
5,
6]; however, placental insufficiency may also contribute to developmental changes [
7]. Maternal immunity and postnatal care may be affected, which may also impact the physiology and behavior of the progeny postnatally [
8]. However, prenatal stress does not always affect the progeny’s physiology, behavior and performance in the postnatal period, which may be partly due to the gestational period in which maternal stress occurs and the stress paradigm used [
3].
Different maternal stress models that simulate housing or management situations or endogenously elevate maternal cortisol, such as repeated adrenocorticotropic hormone (ACTH) injections or administering oral hydrocortisone acetate (HCA), can cause developmental, HPA regulation, behavioral and immunological changes [
3]. Stress responsiveness was increased among progeny born to sows subjected to social or ACTH-induced stress during mid or late gestation [
9,
10] but not early gestation [
11]. Interestingly, HPA regulation is altered when HCA is administered during late, not mid-gestation. Stress-related behaviors, including escape and vocalization, were increased in piglets from sows receiving HCA or ACTH during late gestation [
12,
13]. However, these behaviors were not altered among those born to sows challenged with ACTH during mid-gestation [
14].
Moreover, the gestational period in which maternal stress occurs can also affect the ontogeny and maturation of the immune system of the progeny. One critical window of vulnerability in pigs especially sensitive to maternal stress is the myeloid period, in which the lymphocytic populations rapidly expand between gestational days 60 and 80 [
15]. Progeny born to sows that were subjected to restraint or social stress or administered ACTH during late gestation exhibited altered lymphocyte proliferation and reduced immunoglobulin levels and leukocyte populations, but there was no effect on progeny born to non-stressed sows [
16] or sows experiencing these stressors during early [
11] or mid-gestation [
13]. It is plausible that maternal cortisol may directly affect fetal immune development, as cortisol can pass through the placental barrier [
3] or indirectly affect the immune response of the progeny.
It is plausible that cortisol may disrupt the necessary shift in a Th-1 (cell-mediated) to Th-2 (humoral) phenotype at the maternal-fetal barrier [
17]. Glucocorticoids drive the Th-1 and Th-2 immune bias [
18], thus altering the immune profile in utero, translating to an altered immune profile postnatally [
19]. Another possibility is that cortisol-related effects on the immune response of the dam may affect passive immunity via changes in the immune composition of colostrum and milk, as maternal immunoglobulins were reduced in the colostrum of sows subjected to heat stress in late gestation [
20]. Reduced immune factors in the colostrum and milk may affect the immune development of the progeny. This is especially a concern in species such as swine, where maternal immune cells, immunoglobulins and other large molecules do not cross the placental barrier [
15,
21], making them more vulnerable due to being dependent on colostrum for immunoprotection.
Prenatal stress can affect immunological development and immune function in the progeny and thereby alter the disease susceptibility; however, the outcome may be dependent upon maternal stressor, gestational period, and the experiences of the progeny postnatally. The link between maternal prenatal stress at different stages of gestation on the HPA axis and the immune system of the progeny postnatally is relatively limited. Thus, the current study employed a pharmacological model that mimics prenatal stress in pregnant sows by increasing circulating cortisol concentrations in a controlled manner [
12,
22] via oral administration of hydrocortisone acetate (HCA), a synthetic glucocorticoid, to primiparous sows during mid and late gestation to characterize the stress responsiveness and immune and behavioral phenotypes of the progeny by measuring cortisol, leukocyte populations, cytokines, immunoglobulins and agonistic and oral-nasal-facial (ONF) behaviors. Determining if gestational stress and the period at which stress occurs alter stress and immune measures in the progeny may highlight important time points to minimize maternal stress to maximize the well-being of the progeny.
2. Materials and Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee at Oklahoma State University under protocol no. IACUC-20-19.
2.1. Animals and Experimental Design
A total of 10 Yorkshire × Landrace cross primiparous gilts were artificially inseminated using a single Landrace semen source (DNA Genetics, Columbus, NE, USA). Gilts were kept in standard gestation stalls (0.56 m × 2.36 m) within a mechanically ventilated gestation house at Oklahoma State University Swine Research and Teaching Facility (Stillwater, OK, USA). Pregnancy was confirmed ultrasonically at 38 ± 2 days post-breeding. Gilts were randomly assigned to receive either hydrocortisone acetate capsules (HCA; Spectrum, New Brunswick, NJ, USA), a synthetic glucocorticoid or placebo during gestational days 51 through 72 (mid-gestation; M-HCA n = 3 or M-CON n = 2) or 81 through 102 (late gestation; L-HCA n = 3 or L-CON n = 2). The gilts were hand-fed one HCA gelatin capsule (70 mg/capsule) or placebo (empty gelatin capsule) at 0600 h and another at 1630 h for 21 days. At gestational day 110, all animals were moved and kept in individual farrowing stalls until the end of a 21-day lactation period.
To minimize potential piglet variation due to birth order or uterine placement, during the farrowing process, the first, fourth, and eighth piglets born to each sow were weighed and ear-tagged for identification. The last piglet born that allowed us to balance for sex was weighed and tagged for 4 piglets per litter, resulting in a subset of 36 piglets being used in this study. This subset of piglets remained with their sow and littermates until they were weaned at 21 ± 2 days old. Body weights were recorded at 7, 14, and 21 d post-birth by placing each piglet individually on a digital bucket scale.
Once piglets were weaned, they were moved to a new mechanically ventilated nursery building where they were randomly allotted to same-sex pens balanced for body weight across treatments; thus, each pen housed 2 piglets/treatment from different sows, but the same sex (n = 6 piglets per pen with each treatment be represented). Each pen (n = 6 pens) contained one nipple waterer, and they were fed ad libitum, a standard nursery diet formulated to meet or exceed NRC requirements [
23]. At 42 days of age (or 21 days post-weaning), a subset of piglets were randomly selected and injected intramuscularly with 222 µg of ACTH dissolved in 1 mL of saline (n = 12) or 1 mL of saline (n = 12). Body weights were recorded at 7, 14, and 21 d post-weaning by placing each piglet individually on a digital bucket scale.
2.2. Sample Collection
Colostrum was collected from each sow after the onset of the birthing process but prior to the delivery of the first piglet. Samples were collected by placing two fingers and stripping at least 0.5 mL per teat until at least 5 mL of colostrum was collected from each functional teat. Samples were placed on ice, aliquoted, and stored at −80 °C until further analysis.
Umbilical cord blood was collected prior to suckling from a subsample of piglets at birth. The umbilical cord was placed between two fingers, stripping blood into a vacutainer containing heparin. Blood samples were collected at 7, 14, and 21 days of age, at 24 h and 7 days post-wean, and then prior to ACTH challenge and 1, 2, 4, and 24 h post-challenge. Piglets were held supine, and samples were collected via jugular venipuncture using heparin vacutainers (procedure lasted < 2 min; B.D. Vacutainers; Franklin Lakes, NJ, USA). Samples were immediately put on ice until processing could be completed.
2.3. Complete Blood Cell Count and Lymphocyte Isolation and Proliferation Assay
An aliquot of whole blood was added to an Eppendorf tube for a complete blood cell count (CBC), which was determined electronically using the Element HT5 Hematology Analyzer (Heska, Loveland, CO, USA). Vacutainers were centrifuged at 2100 rpm for 25 min. The plasma was aliquoted and stored at −20 °C until further analysis.
Whole blood was diluted 1:1 with Roswell Park Memorial Institute (RPMI; Gibco, Carlsbad, CA, USA) medium, layered over Histopaque-1077 (density = 1.077 g/mL; Sigma, St. Louis, MO, USA) and centrifuged at 700× g for 30 min at 25 °C. Lymphocytes were then removed, washed twice in RPMI and counted. Cell concentrations were then adjusted to 5 × 106 cells/mL with a solution of 90% RPMI with 10% fetal bovine serum (R10) and placed into the wells of a sterile 96-well flat-bottom plate. Concanavalin A (ConA) and lipopolysaccharide (LPS) were used as mitogens (Sigma, St. Louis, MO, USA) to stimulate T and B cells, respectively, at a concentration of 0, 0.2, 2.0, and 20 μg/mL for ConA and 0, 0.5, 5.0 and 50 μg/mL for LPS. Mitogen concentrations were pipetted into the 96-well plates in triplicate. Then, the plates were incubated for 48 h at 37 °C in a 5% CO2-humidified incubator. From the top of each well, 100 μL was removed and R10 was added; then, the plates were returned to incubation for 18 h. Then, 20 μL of CellTiter 96® AQueous One Solution Reagent (Promega, Madison, WI, USA) was added to each well and the plates were returned to incubation for 4 h. Plates were read using a microplate reader (BioTek Instruments, Winooski, VT, USA) at 490 nm with a reference wavelength of 690 nm. Results are expressed as a proliferation index (P.I.): Optical Density (490/690 nm) stimulated cells–Optical Density (490/690 nm) non-stimulated cells.
2.4. Cortisol, Cytokines, Stress Markers, and Immunoglobulins
Cortisol, cytokine, stress marker, and immunoglobulin concentrations were all measured using a commercially available enzyme-linked immunoassay (ELISA) following the manufacturer’s protocol with minor modifications. Cortisol was measured using kits from Arbor Assays (Ann Arbor, MI, USA) by diluting plasma samples 1:100 and colostrum 1:200. Interleukin-4, -10, and -17, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) were measured using swine-specific ELISA kits from Invitrogen Corp. (Waltham, MA, USA) by diluting plasma samples 1:2 and colostrum 1:10 for IL-4, INF-ɣ, and TNF-α, and at 1:20 for IL-10 and IL-17. Immunoglobulin G (IgG) and A (IgA) were measured using swine-specific ELISA kits from Bethyl Laboratories Inc. (Montgomery, TX, USA) by diluting plasma samples 1:500,000 or 1:10,000 (IgG and IgA, respectively) and colostrum samples 1:1,000,000 or 1:30,000 (IgG and IgA, respectively). Immunoglobulin M (IgM) was measured using swine-specific ELISA kits from Abnova (Taipei City, Taiwan) by diluting colostrum samples 1:20,000. Immunoglobulin E (IgE) was measured using swine-specific ELISA kits from BIOTANG Inc. (Lexington, MA, USA) by diluting colostrum samples 1:20. Corticosteroid-Binding Globulin (CBG) and 11β-Hydroxysteroid dehydrogenase-2 (11β-HSD2) were measured using swine-specific ELISA kits from BlueGene Biotech (Shanghai, China) by using undiluted plasma samples for CBG and diluting plasma samples 1:2 for 11β-HSD2. Corticotropin-releasing hormone (CRH) and Adrenocorticotropic Hormone (ACTH) were measured using swine-specific ELISA kits from FineTest Biotech Inc. (Boulder, CO, USA) by running undiluted plasma samples for CRH and diluting plasma samples 1:5 for ACTH. All samples were run in duplicates.
Plates were read at a wavelength of 450 nm, and a standard curve was generated using a microplate reader (BioTek Epoch; Gen5 Version 3.04.17 Data Analysis Software; Bio-Tek, Winooski, VT, USA) to determine the concentration of the unknown samples. The minimal detectable concentration for cortisol was 27.6 pg/mL, and for Interleukin-4, -10, -17, Interferon-gamma, and Tumor Necrosis Factor-α it was 1.5 pg/mL, 3.0 pg/mL, 14 pg/mL, 2.0 pg/mL and 3.0 pg/mL, respectively. The minimal detectable concentration for IgG and IgA was 1.37 ng/mL. Lastly, the minimal detectable concentration for CBG, 11β-HSD2, CRH, and ACTH was 1.0 ng/mL, 1.0 ng/mL, 1.6 pg/mL, and 12.5 pg/mL, respectively.
2.5. Behavioral Observations
Behavior was captured using color surveillance cameras (Night Owl SP, Boca Raton, FL, USA). Cameras were mounted on the ceiling and positioned over the pen to video record all 6 piglets per pen. Continuous sampling was used to observe and register behavior for 4 h post-weaning and mixing (n = 36 piglets; 6 piglets/pen). Both frequency and duration of agonistic and oral-nasal-facial (ONF) behaviors were registered. The ONF behaviors were further classified as animate (toward conspecific) or inanimate objects (floor, feeder, bars, waterer).
2.6. Statistical Analysis
Data were analyzed utilizing the correlation and mixed model with repeated measures in SAS 9.4 (Inst. Inc., Cary, NC, USA). All traits were tested for departure from a normal distribution through analysis of the residuals; therefore, log transformation was applied to these variables: cortisol, 11β-HSD2, CBG, CRH, ACTH, immunoglobulin G and A, and cytokines. This model included fixed effects of maternal cortisol treatment (HCA or CORT) and gestational period (MID or LATE) for the sow and fixed effects of sow treatment (M-HCA, L-HCA or CON) for farrowing characteristics, colostrum and piglet measures. Significance was set at (p-value ≤ 0.05) with trends discussed at (p-value > 0.05) to (p ≤ 0.10). The Tukey–Kramer adjusted p-value was utilized to analyze farrowing characteristics, colostrum and piglet measures to control for Type 1 Error.
4. Discussion
Maternal stress during gestation can affect the development and responsiveness of the hypothalamic–pituitary–adrenal axis (HPA) and immune system in the progeny. Gestational stage, duration, and type of stressor may also influence the short- and long-term effects on the future progeny. This study revealed differential effects of hydrocortisone treatment (HCA) and days of gestation at which HCA was fed on the immune phenotype and stress biomarkers in the progeny. Consequences of maternal stress occurred at birth, during the suckling and post-wean phases, and in response to an adrenocorticotropic hormone (ACTH) challenge. Piglets born to sows fed HCA during mid-gestation were observed to have reduced measures of humoral immunity, indicated by changes in immunoglobulins and cytokines associated with the humoral immune response, whereas piglets born to sows administered HCA in late gestation were found to have a more robust stress response to farrowing but a dampened or delayed stress response to weaning and ACTH challenge, as evidenced by changes in cortisol and pro-inflammatory cytokine concentrations, leukocyte populations, and stereotypic behavior. The differences in measures of immune and stress between piglets born to sows stressed during mid and late gestation reported within imply that maternal stress and the stage of gestation at which stress occurs can differentially affect immune phenotype and stress responsiveness of the progeny during lactation and 21 days post-weaning.
In utero exposure to elevated maternal cortisol during gestation may be partly attributed to the different immune phenotypes observed at birth and throughout lactation in the progeny. Although the data presented solely reflect the expression of the progeny, combining these results with the lack of difference in colostral cortisol across treatment may indicate that in utero exposure to elevated cortisol levels may have influenced these differences. In utero, exposure to high cortisol levels can impact the development and functionality of physiological systems, including the HPA axis, which undergoes further development later in gestation [
4,
24]. This may partly explain why some aspects of the immune response did not differ regardless of when chronic maternal stress occurred and may account for the higher levels of stress markers like cortisol and neutrophil-to-lymphocyte ratio in the umbilical cord blood of piglets born to late-stressed sows and that remained elevated throughout lactation. However, it is also plausible that these may reflect a more robust stress response to farrowing in the sows. The blood collected from the umbilical cord was a mixture of venous and arterial, indicating that the blood sample was representative of both the sow and her progeny.
It has been established during pregnancy that maternal cortisol remains constant until parturition [
25]. Despite the cortisol increase before the initiation of parturition, changes in the phenotype of the progeny born to sows in this study were absent, suggesting a protective mechanism that controls cortisol levels during pregnancy, especially during vulnerable periods, such as early and mid-gestation, to maintain pregnancy [
26,
27]. One possible mechanism that regulates maternal cortisol is the enzyme 11β-Hydroxysteroid dehydrogenase (11β-HSD2). It has been shown to control cortisol levels during gestational stress in humans, mice, and pigs [
28,
29]. In humans, it was found that lower levels of the 11β-HSD2 enzyme on the placental side resulted in higher cortisol reactivity in children, which may be due to hindering the ability of the 11β-HSD2 enzyme to control maternal cortisol, thus exposing the developing progeny to elevated maternal cortisol [
30]. Reduced placental expression of the enzyme may be associated with excess cortisol exposure, reducing the 11β-HSD2 enzyme’s capacity to convert cortisol to cortisone. Suppose maternal cortisol levels surpass the enzyme’s threshold; in this case, the enzyme’s protective effectiveness is reduced, which could increase the risk of fetal exposure.
Another possible explanation for the observed differences in progeny immune and stress responses could be the increasing levels of progesterone that occur as pregnancy progresses. Progesterone is associated with the retention of pregnancy via the stimulation of Th2 cytokines and the suppression of Th1 cytokines, which can result in pregnancy loss [
31]. Interestingly, it has been noted that progesterone, similar to cortisol, is known to interact and can bind to corticosteroid-binding globulin (CBG). Cortisol-binding capacity may differ by stage of gestation [
32,
33], and progesterone competes with cortisol for binding to CBG [
34]. This may partly explain differences. However, progesterone binds at a lower affinity than cortisol; thus, an increase in free cortisol compared to bound may expose the fetus to excess maternal cortisol. Therefore, if progesterone is bound, this would reduce concentration, which may be attributed to shifts in the Th1/Th2 balance during vulnerable periods of fetal development. This shift might affect fetal exposure to Th1 cytokines, potentially resulting in alterations in immune and stress responsiveness observed at birth and during the suckling period. However, this study did not measure progesterone, so its role remains unknown.
Immunoglobulins do not passively cross the placenta; thus, pigs obtain passive immunity via colostrum consumption [
21]. Immunoglobulin-G (IgG) is the most common antibody for swine passive immunity [
35], with immunoglobulin-A (IgA) being secondary and playing a vital role in mucosal immunity [
36]. Decreased immunoglobulin concentrations in colostrum samples of dams stressed during mid-gestation and decreased IgG and IgA levels in their progeny over the 21-day lactation period may indicate a dampened humoral response. While little work has explored the effect of maternal stress on the pig’s passive immunoglobulin levels, studies observed that IgA levels in milk were reduced in humans and mice exposed to psychological stress during late gestation [
37,
38]. While reduced immunoglobulin concentrations in the piglets may indicate reduced immunoglobulin levels in the colostrum or milk, it has been suggested that increased maternal cortisol at birth may accelerate the neonate’s gut closure in both piglets and calves, which can limit maternal antibody absorption [
39,
40]. However, it is unlikely that decreased immunoglobulin concentrations are due to gut closure. However, initial immunoglobulin differences may be due to alteration during HCA treatment in the dam.
The offspring of mid-stressed sows may have a dampened humoral response. However, those born to late-stressed sows had a more enhanced measure of an immune response, particularly the adaptive immune system. The adaptive immune system, specifically its antibody-producing capacity, can take several weeks of life to mature in the pig [
41]. Thus, measures of adaptive immunity before weaning likely reflect the influence that the sow and her colostrum and milk composition have on the response of her progeny. When comparing the offspring of sows fed HCA during late gestation to those from sows heat-stressed at the same stage of gestation, piglets of late heat-stressed sows exhibited a slower decline in IgG concentration over a 20-day lactation phase [
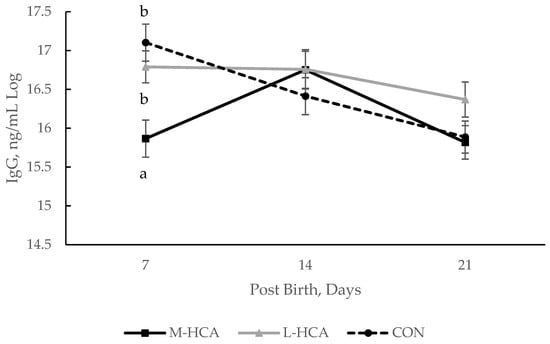
20], similar to the piglets of late-stressed sows in the current study. This, in conjunction with patterns of IgG concentration in piglets of the current study, may indicate that late gestation stress affects the acquisition of passive immunity differently than mid-gestation stress.
Contrary to the reduced measures of humoral immunity, piglets from sows fed HCA during mid-gestation exhibited the highest B cell proliferation at 7 days of lactation, while those born to sows fed HCA during late gestation had higher T cell proliferation at 14 days of lactation and 14 days post-weaning. Couret et al. [
11] similarly found that piglets born to sows that experienced social stress at late gestation had higher T and B cell proliferation at 19 days of age. However, others have found a reduction in the lymphocyte proliferative ability of piglets born to late gestationally stressed sows [
16]. In non-human primates, it has been speculated that prenatal stress reduces the sensitivity of progeny’s lymphocytes to inhibition by cortisol [
42,
43], which may partly explain why reductions in proliferation were not seen in the piglets from treated sows in the current study in response to weaning stress and ACTH challenge. However, this may also be partially due to the delayed or dampened cortisol response in piglets of late-stressed sows.
Multiple studies have found that gestational stress that occurs in late gestation leads to differences in HPA axis organization in the progeny [
4,
10,
44]. Alterations in mRNA associated with ACTH receptors were found in piglets of sows that experienced stress during late gestation [
4,
10], while Kanitz et al. [
44] found a reduction in hypothalamic glucocorticosteroid receptor-binding sites, which may affect the negative feedback mechanism. These alterations may lead to differing physiological and behavioral responses to stressors, as observed in the current study, such as the delay in cortisol peak in response to weaning and the delayed or dampened cortisol response to ACTH challenge in pigs born to dams that received HCA in late gestation. Previous research found that piglets born to sows socially stressed in mid- or late gestation exhibited an elevated and sustained cortisol response to mixing stress [
9], while Kranendonk et al. [
12] found that piglets of sows that were administered HCA in late gestation had lower cortisol responses to ACTH injection than those of piglets from sows administered HCA in mid-gestation or control dams. There is some speculation that ACTH synthesis may be inhibited by monocyte-produced TNF-α [
45], which is particularly interesting, as in the current study, we found piglets born to sows administered HCA in late gestation not only had higher TNF-α and monocyte percentages in response to weaning stress but also increased IL-17, a known recruiter and activator of monocytes [
46,
47]. Though measures of cytokines were not observed during the ACTH challenge, this may provide possible insight into the mechanism of altered stress responsiveness and should be studied further. In contrast, others have found that piglets born to sows exposed to social or restraint stress during late gestation had either reduced or similar pro-inflammatory cytokine levels [
11,
48], which may highlight the differing effect of maternal stressor type on alterations in the progeny.
Finally, maternal stressor type and gestational stage at occurrence may also influence progeny behavioral responses to stressors [
3], which could be attributed to the alteration of ACTH receptors and hypothalamic glucocorticosteroid receptors. Though the current study did not find significant effects of maternal treatment or gestational stage on aggressive encounters, it is noteworthy that there was a decreased time spent in aggressive encounters and the likelihood of inciting or receiving aggressive encounters seen in the piglets of late-gestation HCA-treated sows. This observation aligns with the significant reduction in frequency and duration of observed stereotypic behaviors and a delay in cortisol peak in these piglets. Ison et al. [
49] reported similar findings, with piglets born to sows socially stressed at mid-gestation exhibiting less aggression and overall activity in response to weaning than piglets from control sows. However, consistent with mixed results on cortisol responses, some have found that piglets born to sows administered HCA in mid and late gestation had fewer non-aggressive encounters than their counterparts from non-treated sows [
12].
In contrast, others found that ACTH administration in late gestation resulted in no behavioral changes in response to mixing [
14]. It is plausible that reductions in aggressive and stereotypic behavior seen in the piglets of late-gestation HCA-treated sows in the current study are correlated with the observed delay in cortisol response to the administered stressors. However, the linkage between gestational stress and physiological and behavioral stressor responses in the progeny seems to depend on maternal stressor type and gestational timing. While these findings likely support the speculation of in utero exposure playing a leading role in progeny HPA alteration, even less is known about the effect of abnormal maternal cortisol levels on the development and function of the progeny’s immune response. There is still a gap in understanding how maternal stress during gestation affects the offspring’s immune phenotype and function. These findings highlight that the timing of this stress critically shapes a piglet’s immune response, stress reactivity, and behavior.