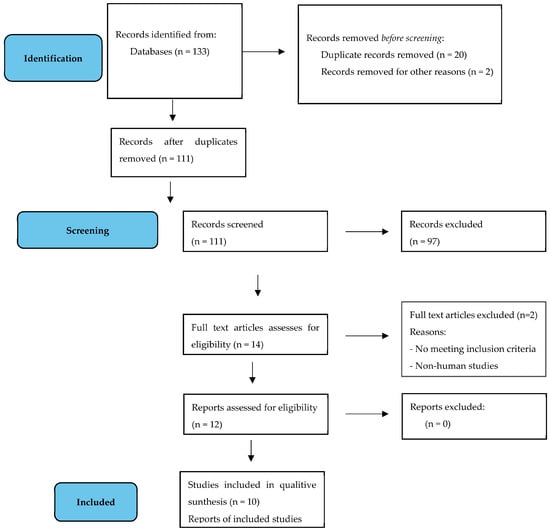
1. Introduction
Premature ovarian insufficiency (POI) is a condition in which ovarian function declines in women under the age of 40, resulting in amenorrhea, elevated levels of gonadotropin-like follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and decreased estradiol levels [
1]. POI can be caused by a variety of factors, including chromosomal anomalies, genetic predispositions, enzyme deficits, autoimmune illnesses, chemotherapy, and viral infection [
2]. Recent research underlines SARS-CoV-2’s potential deleterious influence on ovarian function, stressing its importance for fertility, hormone management, and general reproductive health [
3].
The global COVID-19 pandemic, driven by SARS-CoV-2, has had far-reaching consequences on healthcare systems worldwide. Aside from respiratory symptoms, accumulating data show that SARS-CoV-2 has systemic effects on various organ systems, including the reproductive system [
4]. While the primary focus has been on acute respiratory distress and systemic inflammation, the virus’s possible long-term effects on ovarian function have received little attention [
5]. Premature ovarian insufficiency (POI), which is defined by decreased ovarian reserve and disturbed ovarian function, affects around 1% of women under the age of 40 [
6]. This syndrome is linked to serious reproductive issues, such as infertility, irregular menstrual periods, and an increased risk of osteoporosis and cardiovascular disease. POI can be caused by genetic, autoimmune, and environmental causes, and viral diseases such as mumps and Zika virus have also been linked to ovarian dysfunction. This raises the issue of whether SARS-CoV-2, via its distinct molecular processes, may also contribute to the development of POI [
7].
SARS-CoV-2 affects ovarian function by its ability to enter host cells via the angiotensin-converting enzyme 2 (ACE2) receptor. Early research has identified the ACE2 receptor, critical for SARS-CoV-2 entry into cells, as being expressed in ovarian tissues [
8]. This suggests a plausible direct effect of the virus on ovarian function. Furthermore, emerging studies have linked SARS-CoV-2 to oxidative stress, inflammation, and immune dysregulation, which are key mechanisms implicated in ovarian aging and dysfunction. This receptor is present in multiple tissues, including the uterus, ovaries, and placenta, making these reproductive organs possible targets for viral entry and harm [
9,
10]. The viral spike proteins bind to ACE2 on the cell membrane, facilitating virus entrance and intracellular activity [
10]. One of the negative consequences of SARS-CoV-2 on the ovaries is an increase in oxidative stress. The virus appears to increase reactive oxygen species (ROS) levels in the ovarian environment, disrupting the normal egg maturation process and delaying progression to metaphase II (MII). Additionally, organ hypoxia, as a result of the infection, exacerbates these disruptions, leading to compromised oocyte quality and maturation [
11]. SARS-CoV-2 infection has also been connected to the development of anti-ovarian antibodies (AOAs) in certain women following infection. AOAs have been linked to ovarian failure and unexplained infertility. These antibodies target ovarian autoantigens, which contribute to the development of POI. Case findings reveal that women who previously tested negative for AOAs were confirmed positive following exposure to SARS-CoV-2, further implicating the virus in the etiology of POI [
5,
6,
7].
Furthermore, SARS-CoV-2 infection causes a considerable inflammatory response, as seen by higher levels of C-reactive protein, d-dimers, and pro-inflammatory cytokines such as IL-6, IL-8, and CXCL-10 [
12]. The activation of the NLRP3 inflammasome induces pyroptosis, a type of programmed cell death that impairs cellular homeostasis. Inflammation-induced cell death can negatively impair ovarian function and oocyte development [
13]. Clinically, POI is defined by hormonal abnormalities, particularly high FSH levels greater than 25 IU/L and decreased estradiol levels. Key ovarian reserve markers, such as anti-Müllerian hormone (AMH), inhibin B, and antral follicle count (AFC), are critical for assessing ovarian function and identifying the POI stage. As the illness worsens, it may result in premature ovarian failure (POF), which is characterized by the complete cessation of ovarian activity [
14].
This review sought to consolidate current results on the influence of SARS-CoV-2 on ovarian function, with an emphasis on the molecular processes that may contribute to the onset or worsening of POI, including oxidative stress, immunological responses, inflammation, and hormonal dysregulation. However, the degree and durability of SARS-CoV-2-induced ovarian damage are unknown. While some research found temporary hormonal alterations in COVID-19-affected women, others suggested long-term effects on ovarian reserve and reproductive outcomes. These contradictions highlight the necessity for a comprehensive review of the current evidence.
The primary goals of this systematic review were to assess ovarian function in women exposed to SARS-CoV-2 by looking at both clinical and molecular markers of ovarian reserve and functionality. Furthermore, this review sought to investigate the underlying mechanisms by which SARS-CoV-2 may contribute to the development of premature ovarian insufficiency (POI) and premature ovarian failure (POF), with a focus on oxidative stress, inflammation, and immunological processes. It specifically investigated factors such as oxidative stress, immunological responses, and hormone imbalance, emphasizing their relevance to the development of POI and infertility. This review aimed to give a thorough picture of the virus’s influence on reproductive health by combining these data and identifying areas for future study and therapeutic practice.
2. Methods
This comprehensive review contains research that investigated the effects of SARS-CoV-2 infection on ovarian function, including oocyte quality and hormonal indicators such follicle-stimulating hormone (FSH) and anti-Müllerian hormone (AMH). Eligible studies were clinical trials, cohort studies, and observational studies published between 2020 and 2024. This systematic review has been registered with PROSPERO (Registration ID: 618381) and followed the standards for systematic review protocols.
Studies examining the effects of COVID-19 vaccine on ovarian function were removed in order to focus on the direct consequences of SARS-CoV-2 infection. This choice was made to keep the review’s scope and reduce heterogeneity from research looking at vaccination-related impacts, which might entail diverse processes. While vaccination status was not consistently recorded in the included trials, the outcomes investigated were particularly linked to SARS-CoV-2 infection rather than immunization. We acknowledge this as a shortcoming and propose more study on the influence of vaccination on ovarian function. This review excluded non-English language publications as well as research that lack primary data or fail to address the intended outcomes.
2.1. Data Synthesis
Data from qualifying studies were rigorously retrieved and structured into a comprehensive table that included authorship, research title, sample size, participant characteristics, major findings, and correlations. This organized synthesis makes it easier to compare results from different studies, allowing for a more thorough examination of SARS-CoV-2’s impact on ovarian function and its potential relation to POI.
The variety in research designs, demographics, and evaluated outcomes provided considerable issues due to heterogeneity across the included studies. Differences in participant characteristics, such as age, body mass index (BMI), baseline ovarian reserve, and comorbidities, the timing of SARS-CoV-2 infection relative to ovarian assessment, and variations in measured endpoints, such as hormonal biomarkers, oxidative stress markers, and clinical indicators of ovarian function, were all significant sources of heterogeneity. Furthermore, discrepancies in diagnostic criteria for premature ovarian insufficiency (POI) and study-specific techniques for assessing ovarian function exacerbated the complexity.
To deal with this heterogeneity, a narrative synthesis technique was used instead of a meta-analysis, which would have been unsuitable given the diversity of the data. The studies were divided into thematic categories based on their primary focus, such as hormonal markers (e.g., FSH and AMH), molecular mechanisms (e.g., oxidative stress and inflammation), and clinical outcomes. To allow for meaningful comparisons, data within each theme were further stratified by research design (for example, cohort studies and case–control studies).
Sensitivity studies were performed to investigate potential biases caused by heterogeneity. For example, studies involving severe COVID-19 cases or outcomes collected within 6 months of infection were examined individually to see whether the timing or severity of infection impacted outcomes. Subgroup analyses were also carried out depending on participant age and ovarian reserve status to discover any trends in the data. When contradictions in data were discovered, plausible causes were investigated, such as variations in laboratory tests, sample sizes, or statistical techniques. These processes ensured a fair assessment of the material and identified areas that needed more inquiry.
This review presented a nuanced synthesis of the current research on the impact of SARS-CoV-2 on ovarian function by addressing quality and heterogeneity in an open manner. The methodological framework assures that the conclusions reached are sound, evidence-based, and reflect the wide range of research on this expanding topic.
2.2. Search Strategy
A comprehensive literature review was undertaken using databases such as PubMed, Scopus, EMBASE, and Google Scholar. Search phrases included “premature ovarian insufficiency after COVID-19 infection”, “FSH and AMH levels post-COVID-19 infection”, and “ovarian function following SARS-CoV-2 infection”. To collect the latest and most relevant data, the search included publications published between 2020 and 2024. Reference lists for relevant papers were also reviewed to identify other research that matched the inclusion criteria.
To guarantee methodological rigor and dependability in the included research, a thorough quality evaluation was carried out using proven methodologies customized to distinct study types. For observational studies, the Newcastle–Ottawa Scale (NOS) was used to assess three domains: research group selection, group comparability, and outcome assessment. This scale has a maximum score of nine points, with higher values signifying better methodological performance. Studies with ≥7 points were considered excellent quality, 4–6 as moderate quality, and ≤3 as low quality. The Cochrane Risk of Bias Tool was used to evaluate seven domains in randomized controlled trials (RCTs), including random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, outcome data completeness, selective reporting, and other biases. Based on these criteria, each study was rated as having a low, uncertain, or high level of bias. Discrepancies in rating were handled by discussion among reviewers to ensure consensus.
Table 1 shows the complete findings of the quality evaluation, including individual scores and reasons.
The quality evaluation was important to the synthesis process, ensuring that only research with strong procedures were given significant weight in the analysis and interpretation of data. Lower-quality studies were included to ensure completeness, but their findings were addressed with caution to prevent overinterpretation.
Table 1 summarizes the quality assessments of the papers included in the systematic review. The Newcastle–Ottawa Scale (NOS), which ranges from 0 to 9, was used to evaluate observational studies, with higher scores indicating greater quality. The Cochrane Risk of Bias Tool was used to evaluate randomized controlled trials (RCTs), categorizing studies as low, uncertain, or high risk of bias based on criteria such as randomization, allocation concealment, and outcome reporting. Key comments give more information about each study’s quality and limitations.
This methodology sought to completely examine the available evidence on the effects of SARS-CoV-2 infection on ovarian function, hence providing insights into the probable mechanisms underlying POI and associated reproductive difficulties.
4. Discussion
The results of this systematic review highlight the complex relationship between SARS-CoV-2 infection and ovarian function, which has significant molecular and clinical implications. This study emphasizes the virus’s ability to disturb ovarian homeostasis through processes involving oxidative stress, autoimmunity, inflammation, and hormone dysregulation, all of which are critical to ovarian physiology and reproductive health.
SARS-CoV-2-induced oxidative stress, defined by high reactive oxygen species (ROS) levels, affects oocyte maturation during crucial phases, such as metaphase II arrest, resulting in decreased ovarian function [
4]. ROS destroy vital cellular components such as mitochondrial DNA and proteins, which are required for oocyte competence and energy generation [
23]. Furthermore, hypoxia, a common side effect of SARS-CoV-2 infection, aggravates oxidative stress, making the ovarian microenvironment inappropriate for normal follicular development and ovulation [
15]. This method is consistent with previous findings associating oxidative stress with infertility and ovarian aging, implying that SARS-CoV-2 may accelerate these processes on a cellular level.
The anti-SARS-CoV-2 IgG antibodies found in follicular fluid are mostly linked with natural SARS-CoV-2 infections. These antibodies are thought to alter the ovarian milieu by modifying immunological activation or inflammatory pathways, which might compromise oocyte quality [
24]. While vaccine-induced antibodies are largely of the IgG subtype, they are produced in the absence of the systemic inflammation that occurs with viral infection. There is no indication that vaccine-derived antibodies are present in follicular fluid or that they affect oocyte quality. This discrepancy merits additional exploration, especially considering the widespread use of SARS-CoV-2 vaccinations and their possible impact on reproductive health.
The production of anti-ovarian antibodies (AOAs) following SARS-CoV-2 infection offers compelling evidence of the virus’s autoimmune impact on ovarian health [
25]. These antibodies target ovarian autoantigens, which reduce follicular activity and contribute to POI [
15,
16]. Case reports of AOAs in persons who were previously negative for such antibodies pre-infection suggest a virus-induced trigger mechanism [
15]. Molecular investigations have found common epitopes between viral antigens and ovarian proteins, pointing to molecular mimicry as the fundamental reason for AOA development. This immunological reaction not only depletes the ovarian reserve but also leads to other reproductive issues, such as unexplained infertility [
26]. AOAs inhibit granulosa cell activity, interfering with hormone synthesis and follicular growth, and eventually reducing oocyte quality [
27]. Persistent autoimmunity can also cause persistent ovarian inflammation, fibrosis, and scarring, further decreasing the ovarian reserve and compromising fertility potential over time [
28].
SARS-CoV-2-induced inflammatory responses have a crucial role in the pathogenic consequences on ovarian function. During infection, inflammatory cytokines such as IL-6, IL-8, and CXCL-10 are often elevated [
5]. These cytokines activate the NLRP3 inflammasome, a multiprotein complex that transforms pro-caspase-1 into an active version. Activated caspase-1 induces pyroptosis, a kind of programmed cell death that causes inflammation [
29]. Pyroptosis affects the ovarian follicular architecture and impairs granulosa cell activity, resulting in diminished hormone synthesis and oocyte maturation. Chronic inflammation can lead to fibrosis and scarring of ovarian tissue, reducing the ovarian reserve and reproductive potential [
30].
Hormonal imbalance is another important method by which SARS-CoV-2 affects ovarian functionality. The virus’s systemic inflammation and direct effects on ovarian tissue cause abnormalities in critical hormonal indicators such as FSH, estradiol, and AMH [
6]. Pro-inflammatory cytokines, including IL-6 and TNF-α, impair GnRH production in the hypothalamus, affecting the release of gonadotropins like FSH and LH. Elevated FSH levels seen in some investigations are a compensatory response to reduced granulosa cell activity and decreased ovarian reserve [
31]. Meanwhile, low AMH levels suggest a reduction of recruitable follicles, which raises worries regarding long-term fertility [
32]. AMH, generated by granulosa cells, is an important indication of ovarian reserve, and its reduction demonstrates the virus’s direct influence on follicular health. In some cases, the hormonal changes appear to be temporary; nonetheless, the possible cumulative harm from multiple SARS-CoV-2 infections merits additional examination [
33].
The biochemical structure of follicular fluid is altered, demonstrating SARS-CoV-2’s molecular influence on the ovarian microenvironment [
34]. Lomova et al. found considerable changes in its lipid composition, notably impacting lysophosphatidylcholines [
11]. These lipids play an important role in inflammatory signaling, and their dysregulation is likely to worsen the pro-inflammatory state in the ovary. Furthermore, lower VEGF levels in ovarian tissue, as found in several investigations, may indicate poor angiogenesis [
17]. VEGF is essential for follicular vascularization and oocyte quality, and its inhibition can result in insufficient follicular development and ovulation [
18]. These molecular alterations may account for the reported loss in oocyte quality and reproductive capacity in women recovering from SARS-CoV-2 infection.
The combined impacts of oxidative stress, inflammation, autoimmunity, and hormone dysregulation highlight the complex molecular mechanisms by which SARS-CoV-2 affects ovarian function [
35]. ROS and inflammatory cytokines combine to compromise mitochondrial function and granulosa cell health, whereas AOAs and inflammasome activation aggravate cellular damage [
36]. Together, these pathways decrease follicular growth, deplete ovarian reserve, and may eventually lead to POI [
1]. These molecular discoveries are crucial for determining the long-term effects of viral infections on female reproductive health.
5. Clinical and Research Implications
The findings of this analysis underscore the need of aggressive clinical therapy in reducing the reproductive health consequences of SARS-CoV-2 infection in women of reproductive age. Routine hormonal evaluations, such as AMH, FSH, LH, and E2 levels, are essential for detecting ovarian malfunction early [
37]. Regular monitoring of ovarian reserve using AFC by TVUS and biochemical markers such as AMH should be included in follow-up treatment, especially for women contemplating a pregnancy or receiving ART [
38]. Fertility counseling customized to the individual’s reproductive objectives is critical, including conversations regarding fertility preservation alternatives such as oocyte or embryo cryopreservation, particularly for individuals at risk of POI [
39].
Given the role of systemic inflammation in ovarian dysfunction, anti-inflammatory therapies should be considered for women experiencing prolonged inflammation post-COVID-19. Biomarkers such as IL-6 and TNF-α could guide treatment decisions in these cases. Antioxidants, including CoQ10, vitamin C, and vitamin E, may help minimize oxidative stress and improve the ovarian microenvironment [
40]. Screening for AOAs should be performed in women presenting with unexplained infertility after infection, and immunomodulatory therapies could be explored in cases where autoimmunity is identified [
41].
Individualized stimulation methods should be explored for women undergoing ART to improve oocyte retrieval and quality, especially those recovering from COVID-19. Long-term follow-up care is essential for monitoring hormone profiles and ovarian function, since hormonal alterations may be temporary but can accumulate with repeated SARS-CoV-2 exposures [
42]. Multidisciplinary coordination among endocrinologists, immunologists, and reproductive experts is required to address the multifaceted character of SARS-CoV-2’s influence on ovarian function [
43].
Public health activities should also stress teaching women about the necessity of monitoring their reproductive health following COVID-19 and pushing for immunization as a preventative approach [
44]. These clinical activities highlight the necessity of early intervention and customized treatment in reducing the reproductive hazards associated with SARS-CoV-2 infection.
6. Conclusions
This comprehensive review emphasizes SARS-CoV-2’s diverse influence on ovarian function, with hormonal dysregulation, oxidative stress, and immune-mediated processes as key routes. The findings show that SARS-CoV-2 infection can cause transitory alterations in critical hormonal indicators such as AMH and FSH, whilst molecular disturbances such higher ROS levels and pro-inflammatory cytokines might decrease oocyte quality and ovarian reserve. Although many effects appear to be temporary, the possibility of long-term reproductive repercussions cannot be dismissed.
These data highlight the significance of monitoring and early management for reproductive-age women recovering from COVID-19. Routine evaluation of ovarian function, including AMH, FSH, and AFC, may be crucial in identifying at-risk people and directing fertility planning. Women considering ART should be informed about the possible effects of recent SARS-CoV-2 infection on oocyte quality and IVF results, especially if ART is scheduled within six months of recovery. Furthermore, including hormone measurements into normal post-COVID care for women of reproductive age may assist in reducing the long-term reproductive risks.
For researchers, this review highlights critical gaps in the present literature, such as the need for long-term studies to assess the durability of SARS-CoV-2-induced ovarian dysfunction. Comparative studies on the impact of different viral infections on reproductive health might give a larger perspective, whilst mechanistic research into pathways such as oxidative stress, inflammation, and VEGF dysregulation could help guide focused therapy approaches. Interventions that reduce oxidative stress and modulate inflammatory responses should be investigated to prevent ovarian damage in afflicted women.
Finally, the findings of this research indicate that addressing the reproductive health consequences of SARS-CoV-2 requires a multidisciplinary approach. Prioritizing research and therapeutic care tailored to the requirements of reproductive-age women can help to reduce the long-term effects of COVID-19 on fertility and overall reproductive health.