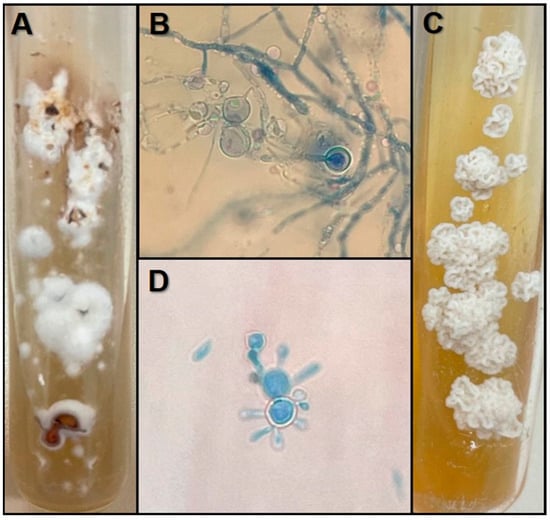
1. Introduction
Paracoccidioidomycosis (PCM) is the most important systemic mycosis in Brazil with high levels of morbidity and mortality. The disease is traditionally associated with rural work, but recent modifications on PCM epidemiology have been described, with the emergence of urban cases of the disease in the Southeastern Brazilian state of Rio de Janeiro [
1,
2]. In 2017, an acute PCM outbreak was reported after a highway construction in Rio de Janeiro state and the agents identified were
Paracoccidioides brasiliensis sensu stricto and
Paracoccidioides americana, suggesting the sympatry of these two species [
3].
Furthermore, PCM geographic expansion has been observed in the Brazilian territory, with increasing cases in the Northern region due to deforestation and land-use dynamics for agriculture commodities [
4]. The main risk factors for PCM infection include middle-aged men, smokers, and working in a rural environment [
5,
6].
The infection is acquired through inhalation of conidia and other infective propagules of the thermodimorphic fungus
Paracoccidioides spp. After reach the pulmonary alveoli, they will convert to the yeast phase and are subsequently phagocytosed by cells of the mononuclear phagocytic system with the formation of the primary pulmonary ganglion complex [
7,
8]. Depending on the host immune status and the inhaled fungal burden and the virulence of the infecting strain, there will be a complete resolution, infection, or lymphohematogenous dissemination and progression to clinical disease [
9]. Therefore, PCM is clinically classified as infection, disease (acute or chronic forms), and residual forms or sequelae [
10]. PCM infection occurs in individuals exposed to environmental sources of
Paracoccidioides, presenting effective cellular immune response, without clinical manifestations [
5]. Chronic PCM is the most frequent clinical form, occurring after a long latency period, in adult patients intensely exposed to soil management activities and affecting mainly the lungs, oral mucosa, and pharynx/larynx. Acute PCM usually occurs in genetically predisposed young patients presenting a humoral response unable to control fungal infection, then quickly evolving with invasive disease characterized by enlarged lymph nodes with liver and spleen involvement [
5,
9].
Regarding PCM case definition criteria, a suspected case consists of clinical and epidemiological features, probable cases being defined by the presence of anti-
Paracoccidioides circulating serum antibodies, while confirmed cases present the identification of the fungal agent in fresh-tissue and histopathological examinations, or culture. The gold standard diagnostic method is culture, and fungal thermal dimorphism is demonstrated through conversion from filamentous to the yeast phase [
11,
12]. Serology plays a key role in the presumptive diagnosis of classic PCM by detecting circulating antigens or antibodies and is suitable for patient follow-up [
13].
In the past, it was believed that
P. brasiliensis was the single species responsible for all PCM cases [
14]. However, advances in molecular identification methods allowed the description of a complex of species, including three phylogenetic species:
P. brasiliensis S1, PS2, and PS3 [
15,
16]. Later, another genetic variant was described as Pb01-like, which, due to its higher genetic and morphologic diversity, was named as
Paracoccidioides lutzii [
17,
18]. The phylogenetic species PS4 was the fifth and last one described [
19].
More recently, whole genome sequencing (WGS) analyses described two clades within the phylogenetic species S1, which were named S1a and S1b [
20]. Thus, according to the Brazilian consensus on PCM,
P. brasiliensis, which is composed of a complex of five phylogenetic species (S1a, S1b, PS2, PS3, and PS4), and
P. lutzii are the species that currently cause PCM [
10]. These phylogenetic species present ecological, epidemiological, and antigenic expression variations, which thus could cause changes in laboratory diagnosis and therapeutic response [
18,
19].
In 2017, a publication evaluated the phylogenetic profile of nuclear and mitochondrial DNA as well as morphological characteristics of the four phylogenetic species of
P. brasiliensis, and proposed considering them as taxonomic species, namely,
P. brasiliensis sensu stricto (S1),
P. americana (PS2),
Paracoccidioides restrepiensis (PS3), and
Paracoccidioides venezuelensis (PS4) [
21].
A more recent publication described two new species of
Paracoccidioides, namely,
Paracoccidioides ceti and
Paracoccidioides lobogeorgii.
P. ceti can be found in dolphins that circulate in oceans with contact with rivers in Latin America, while the latter is found in individuals living in the Amazon Basin and Latin American countries [
22,
23]. These two species, considered uncultivable, can cause PCM loboi and PCM ceti, which are subcutaneous mycoses and may manifest as keloid-like lesions in humans and dolphins, respectively [
12].
Most molecular techniques above described use DNA extracted from fungal cultures and were developed before the recognition of the new species of the genus
Paracoccidioides, thus allowing partial characterizations of the isolates [
11,
14,
21,
24,
25,
26]. Sanger sequencing is the gold standard to identify
Paracoccidioides spp., but this method presents some limitations [
11,
27].
Despite the knowledge of new
Paracoccidioides species, routine diagnosis of PCM is currently based on conventional methods, through identification of the fungal agent in clinical samples and the detection of specific antibodies or antigens in serological tests, while molecular methods have not been implemented in the routine diagnosis of PCM yet, being used as an alternative test [
28,
29]. Furthermore, identifying the fungal species is not required for the clinical and therapeutic management of PCM cases so far [
11].
In 2018, the clinical features and molecular identification of clinical isolates from a cohort of patients with PCM in the state of Rio de Janeiro were described for the first time, with
P. brasiliensis sensu stricto and
P. americana endemic species detected in this region. Until then,
P. americana was considered a rare species, scarcely explored [
30].
Considering the genetic profile occurring in this endemic area, this study aimed to perform a more robust molecular characterization of the species P. americana isolated from clinical samples of patients diagnosed with PCM at a reference center for this mycosis in the state of Rio de Janeiro, from 2015 to 2021, thus evaluating intraspecific variability of these fungal isolates and providing an update of the Paracoccidioides species present in this important endemic area of Brazil.
4. Discussion
For several decades, PCM has been widely identified in Southeast Brazil, Colombia, and Venezuela, but, due to the dense expansion of agricultural frontiers in the Midwest and the Brazilian Amazon during the last 30 years, the epidemiology of this disease has undergone modifications. Therefore, it is fundamental to perform molecular epidemiological surveys in all of those regions [
5]. In fact, Roberto and collaborators [
44] stated the need for genetic surveillance in endemic areas in order to guarantee that molecular epidemiological studies are accurate, due to the epidemiological data recently reported for
Paracoccidioides spp., with the high number of cases in different Latin American countries. Rio de Janeiro is the third state with the highest number of hospitalizations due to PCM in Brazil, and it has recently been facing important epidemiological modifications, such as the description of two endemic species in this state,
P. americana and
P. brasiliensis sensu stricto [
1,
30]. The geographic expansion of PCM cases makes epidemiological surveillance essential to determine which
Paracoccidioides species are present in a given region. Furthermore, through epidemiological surveillance, it is possible to use antigen preparations that include antigens from the prevalent species for a more sensitive serological diagnosis. With a more reliable presumptive result, it is possible to initiate early and appropriate treatment for PCM. Therefore, it is necessary for continuous identification of which species of
Paracoccidioides spp. are recurrently isolated in this endemic area for more accurate clinical decisions to be made in this endemic area.
Similarly to the findings of the previously mentioned study [
30], we found
P. brasiliensis sensu stricto and
P. americana as the species currently circulating in the state of Rio de Janeiro, Brazil. These results, along with the findings regarding the origin of the isolates, may suggest that these species cause autochthonous cases, as most infected individuals are residents and developed risk activities for PCM in the state of Rio de Janeiro. Since 2016—the year of the first publication of a
P. americana case in this state occurred in 2002—it has been possible to suggest that there are two species endemic in this region, sharing similar habitats and producing related clinical characteristics for decades in this endemic area [
30,
34,
45].
The first autochthonous case of PCM caused by
P. americana in the state of Rio de Janeiro refers to a patient who presented the chronic form of the disease [
45]. In addition to this case, five other patients affected by this species were described in this region, all but one presenting the PCM chronic form. The patient with the acute form was diagnosed during the acute PCM outbreak described in this region [
3,
30]. In the current study, all patients infected by
P. americana presented with the chronic PCM form, thus reinforcing a possible tendency of this species to induce chronic presentations in the infected patients.
We previously reported that M13 PCR fingerprinting was a useful tool for identifying molecular types of
H. capsulatum in different geographic regions of Brazil [
46]. However, to date, there are no studies evaluating the intraspecific genetic variability of
P. americana isolates by this method, possibly due to the rarity of this species. However, this species is mostly found in the geographical region herein studied, and allowed the application of this discriminatory genetic test. In this study, we observed that the
P. americana isolates exhibited the same molecular profile. It is noteworthy that the reference strain of
P. americana (Pb03) does not come from Rio de Janeiro, but from São Paulo. Although isolates of
P. americana from these two regions are grouped in different clades through
arf and
gp43 sequencing, showing a possible geographical isolation in the process of species diversification, the reference strain from São Paulo presented, in the M13 fingerprinting, the same molecular profile of the strains from Rio de Janeiro, presenting hypervariable repetitive regions, called minisatellites, similar to those detected in the isolates of our endemic area. On the other hand, isolates of
P. brasiliensis sensu stricto showed a notable intraspecific variability, indicating a higher level of genetic variation, which has been undergoing mutations over the years, perhaps due to the geographic expansion and epidemiological changes regarding the disease in recent years. Interestingly, those two clades of
P. brasiliensis were composed exclusively or predominantly of isolates causing chronic or acute PCM, respectively, which may indicate genetic determinants of the major clinical forms of PCM. A larger study, with
P. brasiliensis isolates from other endemic areas, is necessary to assess this hypothesis.
As well as M13 PCR fingerprinting, there are no studies evaluating the intraspecific genetic variability of P. americana isolates by whole genome sequencing analysis. According to phylogenetic evaluation, there are two distinct populations in the species of P. americana based on whole genome sequencing analysis; the PCA and neighboring tree unrooted of P. americana isolates evidenced the existence of two distinct populations of this species. Considering our results, the seven isolates of P. americana studied were clustered in Clade 1 of the maximum likelihood tree based on the SNPs collected, so both techniques evidenced that there is no intraspecific genetic variability between isolates of P. americana from Rio de Janeiro, and these belong to the same population. Therefore, the Rio de Janeiro population is more diverse than the population of other regions of Brazil.
The analysis of our results indicates that the two methods used in this study are reliable and reproducible for differentiating P. brasiliensis and P. americana. Moreover, these techniques can be used in a standardized approach for typing Paracoccidioides spp. Although sequencing is the gold standard for molecular identification of Paracoccidioides spp., the genetic diversity through DNA microsatellites observed between the two species could allow the differentiation of Paracoccidioides species by fast and inexpensive methods. Furthermore, these advantages are especially valuable in situations where laboratory facilities are relatively limited. For this, more studies are needed, including a larger number of isolates and all species of the genus Paracoccidioides.