The tissue response to disease and injury results in the deposition of a dense scar through processes collectively known as fibrosis [1]. Fibrosis, marked by the excessive accumulation of collagen, is a common pathological feature of various chronic diseases that affect organs such as the liver, lungs, kidneys, heart, and skin [2,3]. Dysfunctional healing by fibrotic scarring results in structural abnormalities and eventually leads to organ malfunction, leading to lifelong disability, which places a substantial burden on public health [4]. Collectively, fibrosis is implicated in 45% of all deaths in the U.S. [5]. As fibrosis is increasingly recognized as a significant cause of morbidity and mortality in many chronic diseases, effective treatments are emerging in certain conditions. For example, liver fibrosis due to HCV can resolve within 12 months of treatment [6], bone marrow fibrosis can significantly improve after stem cell transplantation [7], and SGLT2 inhibitors have been shown to reduce kidney fibrosis in some patients [8]. These advancements highlight the potential effectiveness of targeted therapies in addressing fibrosis; however, such efforts require a comprehensive understanding of the underlying mechanisms to be effectively developed and implemented. In recent years, researchers have explored natural compounds with potential antifibrotic properties. Ursolic acid (UA) has gained attention for its diverse biological activities, including anti-inflammatory, antioxidant, and potential antifibrotic effects [9,10,11,12]. UA is a pentacyclic triterpenoid compound widely found in various fruits and vegetables [11]. Studies have shown UA antifibrotic activity in liver fibrosis models in rats and mice [12,13,14]. The antifibrotic mechanisms of UA likely involve multiple signaling pathways, such as the inhibition of NFκB, PI3K/Akt, Nrf2/ARE, NOXs/ROS, RhoA/ROCK1, and OX4/NLRP3 inflammasome pathways [12,15,16]. However, the antifibrotic effects of UA are complex and somewhat controversial. While some studies have reported antifibrotic effects of UA [11,15,17,18,19], other research has found limited or no significant antifibrotic action [20,21]. Some studies show reduced collagen deposition with UA treatment [11,17,18,19], suggesting potential antifibrotic effects. However, other research indicates that UA can stimulate the production of collagen [20,21,22,23], particularly types I and III, which are major fibrotic proteins in tissue fibrosis. Despite its potential antifibrotic properties, UA is also used in skincare products aimed at reducing signs of aging and improving skin elasticity [22,24], because of its collagen-boosting properties. As such, its impact on skin collagen and fibrosis remains unclear. These conflicting findings highlight the need for further research to better understand the mechanisms and context-dependent effects of UA on fibrosis and collagen production.
Skin provides an excellent model for studying fibrosis [25]. The human skin dermis primarily consists of a dense, collagen-rich extracellular matrix (ECM), which provides structural and mechanical support [26]. Adult mammalian skin wounds typically heal with fibrotic scars, characterized by the excess deposition of abnormally organized, densely-packed collagen fibrils, a hallmark of fibrosis [4,5]. Dermal fibroblasts, the main cellular component of the dermis, play a crucial role in regulating collagen homeostasis.
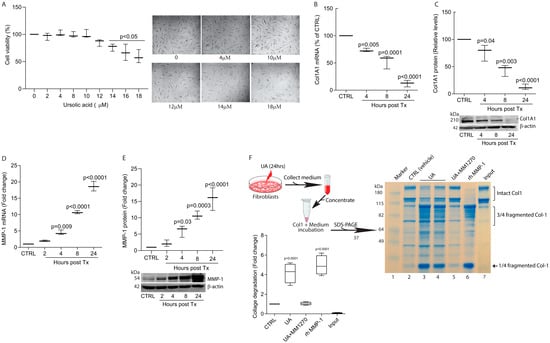
In this study, we investigated the effects of UA on collagen homeostasis in the context of fibrosis using human skin primary dermal fibroblasts. We found that UA significantly inhibits collagen production and induces the matrix-degrading protease, matrix metalloproteinase-1 (MMP-1). Mechanistically, UA impairs TGF-β/Smad signaling to inhibit collagen production and activates mitogen-activated protein kinase (MAPK) pathways and activator protein-1 (AP-1) to induce MMP-1. Finally, we demonstrated that UA prevented bleomycin-induced skin fibrosis in a mouse model. These findings suggest that UA is a potential candidate for the treatment of skin fibrosis.
Source link
Tianyuan He www.mdpi.com