1. Introduction
Population monitoring is a critical component of conservation biology [
1,
2], and understanding the processes that determine population trajectories (i.e., survival and productivity) [
3] is central to the conservation of vulnerable species and to ecosystem management. Long-term monitoring can determine population trends [
4] and enables the evaluation of conservation and management practices and the consequences of unpredictable, catastrophic events such as cyclones or bushfires [
2]. For population monitoring to be effective, estimates must be both accurate and precise, and this can be particularly challenging when animals occur in difficult-to-access or dangerous areas [
5], are widespread, highly mobile [
6], or are sensitive to human disturbance [
7]. Recent technological advances have made remotely piloted aircraft systems (RPAS, hereafter called ‘drones’) more affordable while extending their flight times and increasing payload capacities [
8]. These improvements have been matched by improvements to sensor resolution, and drones are now being widely used in wildlife monitoring [
9].
Australian flying-foxes are widespread, highly mobile species that roost in large numbers, often in inaccessible areas [
10]. Flying foxes are of great importance for forests as they provide invaluable ecosystem services in the form of pollination and seed dispersal [
11,
12], maintaining the genetic connectivity of their food plants over fragmented landscapes. Flying foxes face on-going threats such as habitat destruction [
13,
14], but also the emerging threats of urbanization and associated conflict with humans [
15,
16,
17], range-wide destruction of foraging [
18] and roosting [
19] habitats due to bushfires, and mass die-offs from extreme heat events [
20]. Flying-foxes have slow life histories, with females giving birth to a single pup annually; thus, flying-fox populations have a low intrinsic capacity for increase [
21,
22]. Flying foxes are difficult to monitor due to their extreme mobility [
23] and their habit of roosting, often in large numbers, at inaccessible locations [
10]. In Australia, flying-fox monitoring has traditionally been carried out using ‘on-ground’ methods, where animals are counted by human observers, such as, until recently, under the umbrella of the National Flying-Fox Monitoring Program [
24]. However, that program was highly logistically complex and expensive, and, importantly, the lack of precision of monitoring practices meant that significant population trends could only be detected with sufficient power after more than a decade of range-wide monitoring [
10,
25,
26], limiting the program’s utility for conservation managers to respond with immediacy to emergent population threats (e.g., [
27]). To help address this, our team recently developed a novel ‘from-the-air’ method using drone-borne thermal cameras to count the number of flying-foxes occupying a roost during the day [
28]. This method has been shown to have high accuracy and precision [
27,
28]. However, as with conventional monitoring practices (e.g., [
24]), it does not provide any information on the demographic processes that drive changes in population size. The addition of information on adult survival or productivity allows population modelling to be performed [
21,
22] and provides additional evidence that any observed change in population trajectory has biological, rather than methodological causes (e.g., [
29]).
The biology of
P. poliocephalus, in terms of their arboreal roosting [
30] and strict seasonal breeding [
22] in which a single young is produced per year [
31] and is left in the colony at night [
32], allows for our established methodology for counting flying foxes in their day roosts [
27,
28] to be extended to night-time flights, resulting in a measure of productivity at the colony level. Grey-headed flying-foxes (
Pteropus poliocephalus) are seasonal ‘synchronous’ breeders [
22], with the vast majority giving birth in October [
32,
33]. At approximately 3–4 weeks of age, pups are left in the roost at night while their mothers forage [
32,
33,
34,
35], and they are not capable of independent flight until 3–4 months of age [
33]. Thus, from November to January, the number of pups that remain at a roost site at night can be used to estimate flying-fox productivity on a per-colony basis and at the landscape scale. When combined with a day-time drone flight used to estimate the number of adults in a colony, the estimate of productivity can also provide an estimate of female reproductive performance, which is important for assessments of population health (e.g., [
36]). The grey-headed flying-fox is currently listed as ‘vulnerable’ under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 and by the IUCN [
37]. The National Recovery Plan for the grey-headed flying-fox aims to “Develop robust models of Grey-headed Flying-fox life history and population dynamics, to enable predictions of the likely impacts of threats on population viability”. However, despite the emphasis on population modelling, current recovery management does not include annual assessments of population productivity and individual reproductive performance. The potential for populations to recover after a population perturbation is dependent on the production of young and their successful recruitment into the adult population. Previous assessments of flying-fox reproductive success involved colony surveys, where a minimum of 10 trees in a colony were selected, the sex of adults (as distinguished from this year’s young [
20]) was determined via observation through binoculars (e.g., [
38]), and reproductive output was assessed as the proportion of adult females carrying young [
39]. These surveys have been carried out in the context of roost dispersal (e.g., [
39,
40]) but have not been performed systematically as part of a large-scale program for monitoring. These on-ground assessments of flying-fox reproductive success have important limitations as they can be time-consuming; they only survey a sub-sample of the colony; involve walking through a roost, which may cause disturbance; and they are limited to roosts that are accessible by humans.
Here, we demonstrate a proof of concept where we extend our day-time drone-based monitoring methodology [
27,
28] to survey flying-fox roosts at night to quantify the number of flying-fox pups present in a roost. We go on to combine this with a day-time flight, where the number of adult flying-foxes are counted, in order to assess female reproductive performance. We compare the results with estimates derived from conventional ground-based surveys and discuss how drone surveys can provide an effective new method of estimating flying-fox productivity on a per-colony basis and at a population-wide scale, in a standardized fashion, with low levels of disturbance and at a relatively low cost. We suggest that this new method is an important addition to conventional monitoring practices, and it is needed for estimating flying-fox population trajectories with greater accuracy and precision and for improving our understanding of site-specific impacts (e.g., roost disturbance) potentially affecting productivity locally, as well as broad-scale impacts (e.g., wildfires and extreme heat events) potentially affecting the productivity of the grey-headed flying-fox population as a whole.
2. Materials and Methods
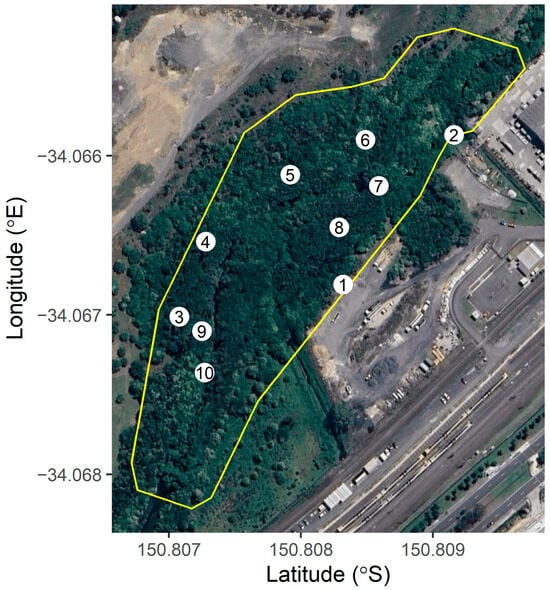
This study was conducted at a flying-fox roost in Campbelltown (−34.067°S, 150.807°E) in New South Wales, Australia. The Campbelltown roost comprises a ~3.5 ha patch of ~15 m tall River Flat Eucalypt Forest vegetation (including Eucalyptus tereticornis, Angophora subvelutina, Bursaria spinosa, and Acacia parramattensis) mixed with non-native plants (including Olea europaea, Cotoneaster glaucophyllus, Ligustrum sinense, and Salix spp.) located along a small permanent creek in a suburban and semi-industrial area. This roost is occupied by a colony of grey-headed flying-foxes (Pteropus poliocephalus) year-round, and from 2019–2023, the size of the colony fluctuated between c. 2980 and c. 15,800 individuals (J.A.W unpublished data). While little red flying-foxes (P. scapulatus) have been occasionally observed in an isolated area of the roost (J.M.M unpublished data), no P. scapulatus were present at the time of the study.
On 15 December 2022, SY conducted a detailed on-ground assessment of the demographic composition of the colony. The area occupied by roosting bats was demarcated by walking the perimeter of the colony with a GPS Kit mobile application (version 8.0.4). To estimate the relative proportion of demographic groups of
P. poliocephalus within the roost, surveys were carried out at a number of ‘observer stations’ distributed throughout the colony according to the relative densities of individuals, ensuring a minimum separation of 500 individuals between each station. At each station, using binoculars, SY observed randomly selected groups (3–5 individuals), identifying age and sex classes (i.e., adult male, adult female, and dependent pup [
20]) until at least 50 individuals were surveyed. This resulted in the identification of the age and sex classes of 520 individuals across 10 observer stations (see
Figure 1). Any flying-foxes that were not dependent pups (i.e., that were older than 1 year) were considered adults. This is because it is hard to distinguish a 1-year-old from a 2-year-old flying-fox (when female flying-foxes are considered to be adults [
41]) without close examination of, e.g., body weight and nipple wear. Sex ratio tests were performed for the whole dataset and for each observation station separately using the ‘sexRatioTest’ function from the R package ‘nipnTK’ [
42].
On the same day as the colony composition assessment was conducted, a DJI Matrice 300 (M300) drone equipped with a DJI Zenmuse H20T radiometric thermal camera (with a combined mass of 7.13 kg) was flown above the colony to obtain thermal imagery of roosting flying-foxes during the day-time (06:14–07:48) and night-time (20:58–22:23). The maximum air temperature recorded in the roost via Kestrel DROP D2 wireless environmental data loggers was 14.4 °C during the day-time flight and 14.6 °C during the night-time flight. The FLIR longwave infra-red thermal sensor in the H20T has a sensitivity of 50 mK @nf/1.0 at a resolution of 640 × 512 pixels.
Following our team’s established procedures [
27,
28], the drone was set to fly at 50 m above ground level (AGL) for all surveys. Though ambient atmospheric conditions affect how much thermal infrared radiation from sources is received by cameras, the effect is small for flights of this height [
43]. Drone surveys were conducted when wind speed was <10 km h
−1. Flights were autonomous from take-off to landing and were conducted in a lawnmower pattern at 2 m s
−1 with 85% front and side overlap above the colony. Thermal orthomosaics were generated using Agisoft Metashape Professional version 1.8.5 (LLC Agisoft, 2019, St. Petersburg, Russia).
The number of flying-foxes present in each thermal orthomosaic was determined via a manual count using the multi-point tool in Fiji 1.8.0_172 [
44]. Previous studies have demonstrated this method to be precise, accurate, and not subject to observer bias [
27,
28]. In the present study, all flying-fox counts from thermal orthomosaics were conducted by the same trained researcher (JM).
This research was approved by Western Sydney University Animal Research Authority no. A15247 and NPWS scientific license SL102047.
4. Discussion
In this proof-of-concept study, we demonstrate that drone-acquired thermal imagery can be used to quantify the number of grey-headed flying-fox pups present in a roost at night when their mothers are away foraging. We further show that when combined with ground observer-based or drone-based surveys conducted during the day, this method allows us to estimate the proportion of adult females with dependent pups. We suggest that our methodology can be used to generate reliable estimates of productivity on a per-colony basis and can provide a practical new means for assessing female reproductive performance, and hence, the size of the effective breeding population (N
e) locally in a standardized fashion and with relatively low effort and cost. When replicated over multiple roosts, this will allow us to monitor population productivity and individual reproductive performance at regional or even range-wide scales. The importance of developing robust models of grey-headed flying-fox population dynamics is highlighted in the National Recovery Plan for the grey-headed flying-fox, and a measure of productivity is essential for this. As well as determining population trajectories (e.g., [
21]), these metrics can be used for assessing population health (e.g., [
36]). Thus, our methodology can yield important new information for informing the sound conservation management of the vulnerable grey-headed flying-fox and other
Pteropus species into the future.
The data from the night-time drone surveys can, in principle, be used to determine the impact of both short- and long-term environmental impacts, as well as management actions, on flying-fox population trajectories. Long-term weather events, such as droughts, have been shown to be associated with a reduction in the proportion of females with dependent pups at local scales [
36]. The current technique can also be used to determine the effects of shorter-term environmental impacts, such as extreme heat events or cyclones. Extreme heat events (when temperatures exceed 42 °C) are predicted to occur more frequently in Australia with human-induced climate change [
46], and these events have a disproportionate effect on adult female and juvenile flying-foxes [
20]. Extreme heat events at a given roost can be forecast several days in advance [
47], and therefore, a drone survey on the night before and the night following an extreme heat event can provide a direct minimum estimate of the number of pups that died during the event. This would allow for a non-invasive assessment of the impact of extreme heat events on the population trajectories of grey-headed flying-foxes. This method can also be used to determine productivity before, during, and after planned management actions, such as cutting buffer zones around flying-fox colonies [
48]. Finally, this method provides a simple way of determining whether a given roost is a ‘maternity roost’ [
32,
49,
50], which has important implications for flying-fox roost management as, for example, the NSW Flying-fox Camp Management Policy [
51] states that roost dispersal is not recommended when “resident female flying-foxes are heavily pregnant until the young can fly independently”.
This study also provides a novel method of investigating the natural history of flying-foxes. For example, an early paper by Nelson [
32] suggested that all pups are left together in the same area, containing well-foliated trees at the edge of a camp. This pattern was not observed in black flying-foxes,
Pteropus alecto [
52], nor in grey-headed flying-foxes [
45], and our study’s results do not support Nelson’s observation either. When the pattern of pups is compared visually to the pattern of roosting adults (
Figure 2), it seems that there are higher densities of pups left towards the center of the colony, and fewer at the edges. This matches the proportion of females seen with pups from the observer stations; for example, the observer stations with the lowest proportion of pups per adult female were stations 1, 2, 4 and 10, which were the closest to the colony boundary (
Figure 1). This suggests that, rather than being moved to a particular location (referred to as a crèche [
32]) when their mothers leave the roost, pups are likely left where their mothers roosted during the day.
Further work is needed to determine the limitations of the current method, including those imposed by the biology of the species, by the technology used, and by financial considerations. One potential biological limitation is that though flying-foxes are seasonal breeders, birthing is not perfectly synchronous. Previous work [
40] suggested that the proportion of adult females with pups plateaus at approximately 3.5 weeks (25 days) after pups are first observed, and since only pups >3–4 weeks old are likely to be left in the colony [
32], we would expect the proportion of pups left in a roost to plateau approximately 7–8 weeks after pups were first observed. This coincides with the time that our night-time survey was performed, but it is possible that if further surveys were carried out, a greater proportion of dependent pups would have been left in the colony, potentially explaining the discrepancy between the expected number of pups (7035, based on the observed proportion of adult females and pups per female from the colony composition survey, 0.57 and 0.78, respectively) and the number observed in the night-time drone survey (6493). Previous work, however, has found the overall sex ratio at grey-headed flying-fox colonies to be 1:1 [
45], with the proportion of adult females increasing towards the centre of the colony [
45]. This increase in the concentration of adult females towards the centre of the colony matches our observations; it is therefore possible that if the whole colony were to be surveyed, no sex bias would be found (e.g., [
45]). This would then result in an estimate of 0.82 pups per adult female present, which is closer to the 0.78 pups observed in the on-ground survey. Furthermore, very detailed on-ground surveys of the adult sex ratio and the ratio of adult females with pups would be required in order to determine whether this is the case.
In addition, neither on-ground surveys of female reproductive success nor this novel method take into account the potential inter-roost mobility of females with pups. Limited catching and tracking data suggest that females with pups > 4 weeks old sometimes move between roosts (J.M.M unpublished data, [
23]); therefore, female and dependent pup mobility may influence estimates of female reproductive success at a given colony. Repeated surveys of the same roost using our novel method would allow short-term changes in the numbers of both adults and pups to be quantified and would therefore provide an estimate of the influence of female mobility on estimates of productivity.
The limitations imposed by technology include the sensitivity with which the drone-mounted thermal camera can differentiate flying-foxes from background vegetation. Drone surveys of the number of adult flying-foxes in their roost have been successfully conducted at <22.6 °C (J.M unpublished data, [
27,
28]), suggesting that the thermal contrast between flying-foxes and background vegetation is still sufficient at air temperatures < 22.6 °C. However, tests have shown that at an air temperature of 24.4 °C, single tree counts from drone imagery led to an underestimation of the true numbers of adult flying-foxes [
28]. As the detectability of a thermal target is a function of the target’s size and ambient temperature (e.g., [
43]), smaller individuals can be expected to be less detectable at even lower temperatures. In addition, high humidity can also reduce the detectability of thermal targets (e.g., [
53]). Therefore, the number of pups present in the roost at night could potentially be underestimated due to the pups’ relatively smaller size, particularly under warmer and more humid conditions. To address this potential limitation, and to maximise the utility of this new method in determining flying-fox productivity, future work should titrate the temperature and humidity combinations for which reliable numbers of pups present can be obtained.
It is possible that animals other than flying-foxes can be detected in an orthomosaic. Previous studies [
27,
28] demonstrated that other animals were rarely observed within a flying-fox roost location, and manual counts of flying-foxes in an orthomosaic (as performed here) rather than semi-automatic approaches provide the opportunity to differentiate between flying-foxes and any false identifications, e.g., due to size or behaviour—e.g., whether birds were roosting or nesting and how birds were grouped together in the canopy. This, however, remains a minor limitation of the current method.
Finally, our method has inherent limitations associated with financial considerations. However, as technology is improving, the cost of drones able to carry out this method has already reduced by about two-thirds (e.g., compare a DJI Matrice 300 with DJI Zenmuse H20T vs. the newer DJI Mavic 3 Enterprise Thermal), and a growing number of environmental professionals (land managers, academics, consultants, and local councils) have the training, experience, and technology to conduct these surveys so that the use of drones and the training of drone pilots do not necessarily require separate expenses. Moreover, when compared to traditional methods of measuring flying-fox productivity (i.e., detailed on-ground colony composition surveys taking up to 8 h), the current method is considerably less time-consuming (c. 1.5 h depending on the size of the colony). Furthermore, unlike day-time drone surveys of flying-foxes that must be conducted in the early morning to maximize thermal contrast between animals and roost vegetation [
28], multiple roosts can be surveyed with a single drone during a single night. Importantly, compared to on-ground observations, the drone-based method arguably also provides better value for money through better-quality data as drone surveys have the advantage of being highly standardized among and within roosts while being relatively very low-impact in terms of flying-fox disturbance [
27,
28], and they also have the advantage of accessing roosts that are inaccessible by foot.