1. Introduction
The increasing global warming and the frequent occurrence of extreme weather events, driven by greenhouse gas emissions over the past few decades, have compelled scientists to seek alternative materials that not only fulfill human needs but also minimize adverse environmental impacts [
1]. In contrast to fossil fuels, biomass is a renewable resource that can be cultivated and harvested in an eco-friendly and sustainable manner [
2]. Furthermore, biomass is characterized as a carbon-neutral resource, meaning the carbon dioxide emitted during its combustion is precisely counterbalanced by the carbon dioxide absorbed by plants during their growth process, thereby ensuring a neutral impact on the atmospheric carbon balance [
3]. Therefore, biomass is considered an ideal alternative material for environmental protection, such as the preparation of nanosilica.
Nanosilica, characterized by its distinctive ultrafine nanoscale dimensions (1–100 nm), displays an array of remarkable characteristics and has been broadly utilized across numerous domains, such as coatings [
4], cementing materials [
5], catalysis, drug delivery [
6], and water and wastewater treatment [
7]. The gas-phase, precipitation, and sol–gel methods [
8] are the conventional methods for preparing nanosilica. These methods often require expensive reagents and silica precursors, such as silicon tetrachloride, tetraethylsilicate (TEOS), sodium tetramethylsilicate, and sodium silicate [
9], which are toxic and ecologically harmful. In addition, high temperatures are required in the gas-phase method and in the preparation of precipitation precursors of sodium silicate, which makes these processes energy-intensive, costly, and emits large amounts of carbon dioxide [
10]. Therefore, the synthesis of nanosilica from safer, cheaper, and environmentally friendly silicon-containing waste is an encouraging trend.
Biomass wastes, especially their ashes, are recognized as promising raw materials for nanosilica production owing to the consistent generation of ash and its substantial silica content. With a high content of natural silica, rice husk is the most thoroughly researched biomass precursor material for the synthesis of amorphous nanosilica. Rice husk is an abundant agricultural waste that provides a sufficient source of raw materials for the preparation of nanosilica. The estimated global rice production was approximately 534 million tons in 2024 [
11], which resulted in approximately 106 million tons of rice husks. In addition, the utilization of rice husks can reduce the accumulation and incineration of agricultural waste, thereby lowering environmental pollution [
12]. Numerous investigations have been carried out to extract nanosilica from rice husks.
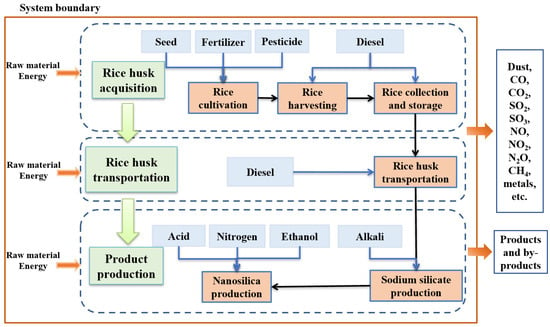
Typically, the synthesis of nanosilica from rice husk is reported to involve thermal and chemical methods [
9]. Low ash production capacity, non-uniformity of ash products, and time-consuming operation (i.e., batch-like operation) are the major disadvantages of the thermal method [
13]. Chemical extraction has the potential to considerably minimize metallic contaminants and enhance the quality of bio-silica compared to the direct thermal method [
14]. Therefore, scholars favor the chemical method more. In the chemical method, rice husk is washed and dried to remove impurities, and then rice husk ash is prepared through acid leaching and thermal treatment. Subsequently, a silicate solution is obtained via alkaline treatment, which is then precipitated in an acidic medium to synthesize nanosilica. The alkaline treatment is performed by the addition of an alkaline solution, such as sodium hydroxide (NaOH, primarily) [
15,
16], sodium carbonate (Na
2CO
3) [
17], or potassium hydroxide (KOH) [
18]. The precipitation reaction is performed using hydrochloric acid (HCl) [
19,
20], sulfuric acid (H
2SO
4) [
21,
22], phosphoric acid (H
3PO
4) [
23], and nitric acid (HNO
3) [
24]. However, previous research has mainly focused on the preparation process of rice husk-derived nanosilica (RH nanosilica) or the development of its applications. In contrast, there is a notable lack of literature that systematically evaluates the environmental impact of RH nanosilica prepared using the chemical reagents mentioned above from an environmental perspective.
Life cycle assessment (LCA) serves as a powerful instrument, empowering enterprises to gain a thorough understanding of the environmental footprint of their products and operations, thereby steering them toward informed decision-making that is aimed at alleviating these impacts. It constitutes a comprehensive methodology for assessing the environmental repercussions associated with every stage of a product’s life cycle, encompassing raw material extraction, production, utilization, and eventual disposal or recycling. LCA has achieved broad acceptance across a diverse range of industries. For instance, Arfan et al. [
25] performed an environmental and economic assessment of hydrogen production from biowaste and biomass from a life cycle perspective, with a high degree of primary life cycle inventory data on materials, energy, and investment flows. Dominic et al. [
26] synthesized nanosilica from millet husk, and they indicated that millet husk nanosilica has a lower environmental impact when compared to rice husk nanosilica. Zhao et al. [
27] used a consistent LCA methodology to show how the type of yeast (C6 or C6/C5) used for ethanol fermentation, as well as the type of acid (H
2SO
4 or CO
2) used to neutralize CaO pretreated slurry, affects environmental and human health aspects.
However, to date, no comprehensive LCA studies have been published regarding the production of RH nanosilica, and only LCA analyses of specific processes are available. For instance, Joglekar et al. [
28] performed an LCA of rice husk nanosilica prepared by NaOH and H
2SO
4 and observed a total climate change of 7.26 kg of CO
2 equivalent per kg of silica production. In fact, various acids and alkalis have been used to prepare nanosilica from rice husks, but a preparation process that is more beneficial to the environment has not been studied. This paper innovatively conducted a comparative and comprehensive analysis of existing preparation processes for RH nanosilica, evaluating them from an environmental perspective.
The objective of this study was to employ the LCA method to demonstrate how the type of alkali (NaOH or Na2CO3) that is used for extracting water glass from rice husk, as well as the type of acid (HCl, H2SO4, or HNO3) which is used for precipitating water glass to nanosilica, affect the environmental emissions of RH nanosilica. Six nanosilica production scenarios were explicitly compared to identify the most environmentally friendly route. Furthermore, the preferred route underwent further in-depth optimization, aiming to achieve optimal environmental emissions for RH nanosilica. The impact of KOH on the environmental emissions of nanosilica was not taken into account in this paper as no emission data for KOH were available in the software database. Similarly, due to the complexity and uncertainty of the byproducts resulting from the H3PO4 treatment process, the H3PO4 production scenarios were also excluded from consideration in this study. This study provides a theoretical foundation for the sustainable development and low-carbon evolution of RH nanosilica. The results are important for the promotion and guidance of the industrialization process of deriving nanosilica from rice husk and accelerating its commercialization.
5. Conclusions
In this study, a comparative LCA was conducted to evaluate the environmental impact of different alkaline extraction (NaOH and Na2CO3) and acid precipitation processes (HCl, H2SO4, and HNO3) on RH nanosilica emissions and to determine the most eco-friendly route. Further in-depth optimization was conducted on the preferred route, aiming to achieve optimal environmental emissions for RH nanosilica. The key findings are as follows:
Compared to Na2CO3, using NaOH resulted in significant decreases in five indicators (AP, EP, POCP, GWP, and ADP fossil) and significant increases in only two indicators (ODP and MAETP). Compared to H2SO4 and HNO3, the HCl scenario exhibited the lowest emissions according to seven indicators (ADP fossil, TETP, GWP, AP, EP, ODP, and POCP) and the highest emissions based on the MAETP indicator.
The results indicate that NaOH and HCl are better selections for the chemical method to prepare nanosilica from rice husk. However, there are some challenges that need to be addressed in the usage of NaOH and HCl due to the higher MAETP criteria. During production, wastewater from NaOH and HCl should be deeply treated with technology to meet environmental standards before discharge or recycling. Enhancing alkaline and acidic wastewater reuse technologies will fundamentally mitigate the harm to marine ecosystems.
Green electricity sources, such as wind, hydropower, solar power (both photovoltaic and thermal), and biogas electricity, can significantly reduce the environmental emissions of the nanosilica life cycle, achieving a 20–60% reduction in environmental impacts. Enterprises can enhance the integration of green electricity into production to reduce emissions and support sustainability.
To reduce diesel emissions, there is a need to decrease diesel consumption by optimizing designs, using efficient generators, and improving fuel management. Alternatively, developing clean fuels, such as biodiesel, can theoretically help lower emissions.
The most effective approach to reducing fertilizer-related and pesticide-related emissions is to enhance their utilization efficiency and decrease their consumption without compromising crop yields. Possible measures to enhance fertilizer utilization efficiency include applying fertilizers in appropriate amounts, at deeper soil levels, or with higher precision, along with using bio-fertilizers and organic fertilizers, and adopting improved fertilization methods, such as drip irrigation. By selecting eco-friendly biological pesticides, optimizing pesticide application methods, promoting the agricultural circular economy, strengthening pesticide management, and adopting low-carbon agricultural technologies, effective reductions in carbon emissions from pesticides can be achieved.
A notable limitation encountered in this study was the exclusion of real-time transportation data, which may have impacted the comprehensiveness of the findings. Furthermore, the research highlighted in this paper focuses on environmental impact analysis. Nevertheless, in practical industrial production processes, it is crucial to comprehensively take into account product quality, production costs, and environmental emissions. Consequently, economic analyses of RH nanosilica will be undertaken in future research endeavors.