1. Introduction
The intestine mainly comprises the mucosal layer and the lamina propria including epithelial cells, immune cells, and tight junctions. It is not only an important part of absorbing and digesting nutrients but also the largest immune organ [1,2]. A healthy intestine is critical for the disease defense, overall metabolism, physiology, and growth of piglets [3] The intestinal epithelial cells serve to absorb nutrients, which play a crucial role in preventing antigens from entering blood circulation by involving in the formation of tight junctions [4]. Therefore, the intact intestine is important for piglets to maintain normal life activities [5]. The small intestine as the longest digestive system is essential for nutrient digestion and absorption. In addition, various bacteria parasitize the intestine, which is divided into beneficial bacteria, intermediate bacteria, and harmful bacteria. When the intestinal environment is healthy, these three types of bacteria are in a dynamic equilibrium state [6]. The gut microbiota is important for piglets to develop the immune system and structure of the intestine [7]. However, as intestinal function and structure are not yet fully developed, piglets are susceptible to the stimulation of various external environmental factors, which will influence intestinal function and structure and lead to intestinal bacterial dysbiosis [8,9,10,11]. An imbalance of gut microbiota causes the translocation of gut microorganisms, resulting in the weakened function of the intestinal barrier, excessive production of pro-inflammatory cytokines, and excessive activation of the intestinal mucosal immune system, which in turn together promotes the development of inflammatory bowel disease [12,13,14]. Therefore, it is important for the growth and development of piglets to maintain the balance of gut microbiota.
However, the pathogenic microorganisms seriously hinder the healthy development of piglets. ETEC is one of the common bacterial pathogens that seriously threaten the intestinal health of piglets [15]. ETEC can produce one or several enterotoxins causing secretory diarrhea [16]. ETEC mainly causes diarrhea in piglets, leading to higher incidence and mortality rates, reduced growth performance, and significant economic losses in the pig industry. A study has shown that ETEC is capable of causing a systemic or localized immune response, which will cause the excessive release of inflammatory cytokines and damage intestinal cells or tissues [17]. Adhesins are proteins that can play a significant role in bacterial colonization and are mainly composed of proteins or phospholipids, usually found in the cell walls and pili on the surface of bacteria. The common pili adhesins in ETEC include F4 (K88), F5 (K99) and others. One of the biggest threats to piglet intestinal health is ETEC K88. Firstly, ETEC adheres to the intestinal epithelial cell receptors of piglets by adhesins. Subsequently, ETEC can avoid shedding caused by intestinal peristalsis and stably colonize. Finally, ETEC destroys the intestinal epithelial cells by secreting heat-labile enterotoxins (LT)and heat-stable enterotoxins (ST), which will cause water and electrolyte disorders in the body, ultimately leading to diarrhea [18,19,20]. Therefore, it is important to find green nutritional modifiers to prevent or treat intestinal damage in piglets caused by ETEC K88.
Zinc (Zn) is a key trace element in animal nutrition, which not only participates in cell proliferation and differentiation but also maintains cell membrane integrity and regulates protein metabolism in the body [21,22]. When Zn is deficient, animals usually show symptoms such as decreased feed intake, reduced feed conversion efficiency, and accompanying [23]. Zn can be added to feed in many ways, with ZnO being the most common, and ZnO is important for weaned piglets to control diarrhea and improve growth performance. Previous study has shown that ZnO in feed improves several digestive enzyme activities in the pancreatic tissue of pigs, improves intestinal morphology, and consequently improves the digestion and absorption of nutrients [24,25]. Cytokines can be directly or indirectly involved in immune-inflammatory response reactions. Previous research reports showed that ZnO treatment improved the expression level of inflammatory factors (TGF-β) by using piglet intestinal epithelial cells (IPEC-J2) infected with E. coli F4 as an in vitro research model, suggesting that ZnO treatment inhibited the inflammatory response caused by ETEC K88 infection [26,27]. Recent reports have shown that ZnO plays a very important role in animal production, which not only can improve immunity function and enhance antioxidant properties, but also reduce diarrhea rate and improve growth performance. However, the influences of ZnO on the damage of the intestine of piglets infected with ETEC are not fully understood. The inclusion of ZnO in medicated pig feed, which is utilized to treat neonatal diarrhea and other health issues in piglets, is prohibited in several countries, including those in the European Union, due to concerns regarding environmental and public health, but in China, the maximum allowable dose of zinc oxide for use in piglets within the first two weeks after weaning is 1600 ppm. The purpose of this study is to evaluate the impact of ZnO (100 mg/kg BW) on intestinal function and growth performance in ETEC K88-infected piglets.
4. Discussion
Zinc (Zn) as an essential trace element plays an important role in maintaining the physiologic function, promoting metabolism, and ensuring healthy growth in animals [41]. The zinc required by animals can be supplemented in the form of ZnO. Previous studies have demonstrated that ZnO significantly influences the growth performance of animals and is closely related to the metabolism of certain nutrients. It can also improve the intestinal mucosal morphology of livestock and poultry, alleviate diarrhea, enhance immune function, and boost antioxidant enzyme activities [42,43]. Nonetheless, the effect of ZnO on the structure and function of the intestine and gut microbiota in piglets infected with ETEC K88 remains unexplored. The use of ZnO may effectively mitigate the symptoms of ETEC infection in piglets, potentially leading to a reduction in economic losses within the pig industry. This study showed that the ZnO administration alleviated diarrhea caused by ETEC K88 infection by improving intestinal morphology, restoring redox balance, reducing apoptosis of intestinal epithelial cells, and correcting gut microbiota dysbiosis in piglets.
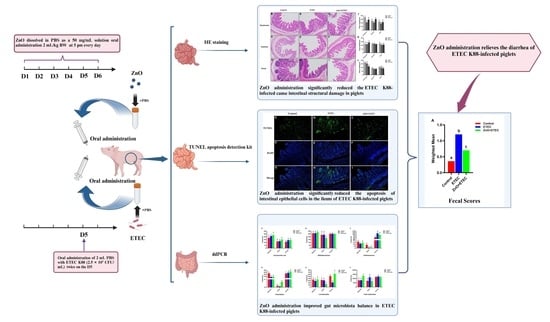
ETEC K88 infection often results in watery diarrhea and reduced food intake, which can lead to intestinal damage in 7 to 15-day-old piglets [17,44]. Previous research has demonstrated that ETEC K88 decreased the natural barrier defenses of the intestinal mucosa and impaired intestinal mucosal function [45,46]. This study has shown that ZnO administration significantly improved the fecal scores of the ETEC K88-infected piglets (Figure 1), which provided new evidence for the positive efficacy of ZnO on the intestine of piglets infected with ETEC K88. Diamine oxidase (DAO) is stable in peripheral blood and is an intracellular enzyme in the small intestinal mucosa of mammals. Once the intestinal mucosa is damaged, the DAO in the small intestinal mucosa is released into the blood [47,48]. When the intestinal absorption function is impaired, the ability of the intestinal lumen to absorb D-xylose into the portal vein is impaired, which consequently lowers the level of D-xylose in the blood. Therefore, the intestinal mucosa integrity can be indirectly reflected by the activity of DAO and the level of D-xylose in the plasma, which is a key indicator for monitoring the intestinal barrier function [49,50]. Compared to the ETEC group, the activity of DAO in plasma in the ZnO + ETEC group was significantly reduced in this study (Figure 1). In addition, the intestinal epithelial cell renewal begins with a pool of intestinal mucosal crypt stem cells that form a new monolayer of epithelial cells, which then migrate to the intestinal villi and develop into mature cells capable of absorbing nutrients [51]. Therefore, the height of the intestinal mucosa villi is a key indicator of the gut health. The invasion of pathogenic microorganisms can accelerate epithelial cell regeneration, leading to the shortening of villi in the intestine [52]. The higher height of the villi means the more epithelial cells, the more surface area, and the better intestinal absorption of nutrients [53]. In this study, ZnO administration reduced the intestinal damage in ETEC K88-infected piglets by increasing the height of villi in the ileum, jejunum, and duodenum (Figure 3). In short, ZnO administration can alleviate the ETEC K88 infection-caused intestinal damage to relieve the piglet diarrhea.
Research has indicated that ETEC K88 may induce significant intestinal stress in piglets, including both oxidative and immune stress, which results in harm to the intestines [54,55]. The purpose of this study is to study the mechanism by which ZnO reduced intestinal damage in the ETEC K88-infected piglets, by measuring the redox levels of the intestine in the infected piglets. It is an important role for enzymatic and non-enzymatic antioxidant systems to maintain normal cellular activity in the organism, which can maintain a balance between pro-oxidant and antioxidant systems [56]. Intestinal oxidative stress induced by ETEC K88 infection is a key contributor to intestinal damage in piglets [57,58]. In the present study, ZnO administration enhanced the intestine antioxidant capacity of the ETEC K88-infected piglets (Figure 4). The T-SOD and CAT activities in the jejunum and T-SOD activity in the ileum were significantly increased after ZnO administration. Additionally, the level of MDA in the ileum and the content of H2O2 in the jejunum were significantly reduced, with a decreased trend observed in H2O2 content in the ileum (Figure 4). These results showed a new finding by which ZnO attenuated ETEC K88-induced intestinal damage in piglets. IL-1β is a key member of the IL-1 family, which has garnered attention for its important role in inflammation-related diseases59 [59]. IL-1β exhibits the activity of potent pro-inflammatory and promotes the secretion of various pro-inflammatory mediators, including chemokines and cytokines [60,61]. IL-1β, as a potent pro-inflammatory, is essential for the host defense response to infection, which is involved in the host response to a wide range of antigens and promotes the expression of inflammatory factors [62,63]. The mRNA level of IL-1β in the ileum was remarkably up-regulated after ETEC K88 infection (Figure 5B), which indicated that ETEC K88 infection stimulated the inflammatory response of the ileum in piglets in this study. However, the administration of ZnO remarkably suppressed the mRNA level of IL-1β in the ileum of the ETEC K88-infected piglets. In order to further study the underlying mechanisms, the apoptosis levels of intestinal epithelial cells were evaluated. The apoptosis levels of the ileal epithelial cells of piglets were increased after ETEC K88 infection; however, the addition of ZnO significantly reduced these apoptosis levels in piglets infected with ETEC K88 (Figure 6). Apoptosis is a form of programmed cell death, which plays a crucial role in maintaining the normal morphology and function of the intestine. The homeostatic imbalance between cell proliferation and apoptosis can adversely affect the normal structure and functionality of the intestine [64]. In summary, administering ZnO may help reduce the damage to the intestine in the ETEC K88-infected piglets. This is achieved by lowering the mRNA levels of IL-1β in the ileum and maintaining a balance between the apoptosis and regeneration of ileal epithelial cells.
The piglet intestine hosts a microflora that interacts symbiotically with the host. This microflora constitutes the microecological balance in the piglet intestine, which is essential for various physiological processes, including growth and development, disease resistance, and nutrient metabolism. Consequently, there has been an increasing focus on gut microflora research [65]. For instance, Enterococcus effectively inhibits intestinal pathogens and helps maintain intestinal health. Some studies have shown that Enterococcus can inhibit chlamydia and parasites, as well as prevent intestinal infections caused by protozoa [66,67,68]. Clostridium can alter the distribution of lymphocytes within the colorectal epithelium and promote the accumulation of Treg cells in the colonic mucosa, causing an increase in the levels of TGF-β in the colon [69,70]. Lactobacillus interacts with the immune system, selectively allowing Lactobacillus colonization, which helps regulate immune responses [71]. Additionally, Lactobacillus stabilizes the barrier function of the intestine by various mechanisms, including the promotion of mucus secretion and the upregulation of the levels of tight junction proteins [72]. The intestinal flora can be influenced by a whole range of factors, for example, genetics, host age, diseases, and environmental conditions [73,74,75]. The unstable gut microbial community is one of the primary causes of diarrhea for lactating piglets [76]. Therefore, it is vital to avoid the effect of external factors on the composition of intestinal flora to promote the health of the intestine in suckling piglets. Previous reports have indicated that low doses of encapsulated ZnO (500 mg Zn/kg) could modulate gut flora and improve the number of beneficial bacteria in the intestinal tract, reducing diarrhea in weaned piglets [77]. ETEC K88 notably increased the counts of Enterococcus in the cecum and the counts of total eubacteria in the ileum, while significantly decreasing the counts of Enterococcus and Clostridium in the ileum, the counts of Lactobacillus in the jejunum, and the counts of Lactobacillus in the cecum of piglets in this study. The above results suggested that ETEC K88 infection led to dysbiosis of intestinal flora in piglets. However, the addition of ZnO reversed the increases in the counts of Enterococcus in the cecum and the counts of total eubacteria in the ileum, as well as the decreases in the counts of Clostridium in the ileum, the counts of Lactobacillus and in the jejunum, and the counts of Lactobacillus in the cecum after ETEC K88 infection. In a word, incorporating ZnO into the feed alleviated the structural dysregulation of intestinal flora in the jejunum, ileum, and cecum of piglets infected with ETEC K88.
Source link
Yanyan Zhang www.mdpi.com